Abstract
Purpose
One of the great conundrums for both oncologists and cardiologists is how to best monitor the potential and actual cardiotoxicity of doxorubicin. Pegylated-liposomal doxorubicin (PLD) has a safer cardiotoxicity profile than bolus administration of doxorubicin. Although ejection fraction (EF) is commonly performed to monitor doxorubicin-induced cardiotoxicity, evidence for its predictive utility is limited. We examined the incidence of doxorubicin-induced heart failure (HF) in patients who received a large cumulative dose of doxorubicin as PLD and its relation to EF and HF.
Methods
A retrospective chart review of patients who received a large cumulative dose of PLD, sometimes after previous free doxorubicin treatment, was performed to examine the incidence of doxorubicin-induced heart failure (HF) and its relation to EF and development of HF.
Results
No definite doxorubicin-induced clinical HF was observed among 56 patients (median age 54; 15–93) who received a cumulative doxorubicin dose (free + PLD) of >450 mg/m2. Of these, 49 received >500 mg/m2, 28 > 700 mg/m2, 19 > 800 mg/m2, 14 > 1000 mg/m2, and 5 > 1400 mg/m2. The EF varied greatly over time in some patients treated with PLD in the absence of symptoms or signs of heart failure, and was not particularly useful in making decisions regarding further dosing.
Conclusions
Pegylated-liposomal doxorubicin was associated with a low risk of doxorubicin-induced HF in a retrospective cohort of patients receiving large cumulative doses of doxorubicin and long-term follow-up. EF did not predict doxorubicin-induced cardiotoxicity in our cohort of adult patients receiving PLD. Given the lack of prognostic clarity regarding modest EF changes, regular EF monitoring may not be warranted, at least when PLD is used in adults. Modest changes in EF should probably not be used to limit a patient’s access to PLD, but may warrant cardiology consultation for long-term follow-up after completion of therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the great conundrums for both oncologists and cardiologists is how to best monitor the potential and actual cardiotoxicity of doxorubicin. Despite its efficacy, the use of doxorubicin is limited by its dose-related cardiotoxicity, which may manifest as a reduction in ejection fraction (EF) and/or development of clinical (HF). While several risk factors for doxorubicin-induced heart failure (HF) have been reported, the cumulative dose administered appears to be the most important one [1]. Retrospective studies have found a ~7.5% incidence of clinical HF at a cumulative dose of 550 mg/m2 of free doxorubicin [1], and prospective trials have found even higher rates [2]. In one large series, the incidence of HF rose to ~20–30% at 700 mg/m2, reaching ~50% in the setting of prior cardiovascular disease, and to ~50% at 1000 mg/m2 with no prior cardiovascular disease [1]. However, the diagnosis of HF is typically a clinical exercise (i.e., bedside) and is subject to error [3].
The cardiotoxicity of doxorubicin appears to correlate with peak plasma levels and total cumulative dose [4]. A weekly schedule of administration is associated with a lower incidence of HF than an every 3-week schedule [1], and continuous intravenous infusion (CIVI) of doxorubicin is associated with less cardiotoxicity than bolus administration [4,5,6,7,8,9,10].
Pegylated-liposomal doxorubicin (PLD) is a liposomal formulation of doxorubicin in which the doxorubicin is contained in liposomes that are coated with methoxypoly (ethylene glycol). The methoxypoly (ethylene glycol) coating results in less uptake by the reticuloendothelial system and a much longer half-life in blood than non-pegylated liposomes [11,12,13]. The toxicity profile of PLD is more similar to that of CIVI doxorubicin than bolus administration of doxorubicin [9,10,11]. The long intravascular half-life of PLD markedly limits peak doxorubicin exposure to the myocardium [11,12,13]. Animal studies found less histologic evidence of myocardial damage with PLD relative to free doxorubicin [14, 15], and human studies suggest the same results [11, 13, 16,17,18,19,20]. Studies in Kaposi sarcoma found lower endomyocardial biopsy scores in patients treated with PLD (near normal scores) as compared with free doxorubicin [16]. Two studies in breast cancer found PLD had less cardiotoxicity than doxorubicin [17, 20]. Retrospective studies of PLD treatment found no clinical HF attributed to PLD among 42 patients who received ≥500 mg/m2, 116 patients who received ≥400 mg/m2, or 22 patients who received >550 mg/m2 [21,22,23]. Phase II trials of PLD patients with sarcoma or mesothelioma found no definite cardiotoxicity in 18 patients who received more than 500 mg/m2, 10 of whom received >700 mg/m2 [18, 19]. A retrospective study in gynecological cancer found only 3 of 53 patients at high risk of doxorubicin-induced cardiotoxicity developed HF possibly related to PLD [24].
Because doxorubicin is a highly effective anti-neoplastic agent, and doxorubicin-induced HF is a serious toxicity, a number of approaches have been used to predict the development of doxorubicin-induced HF. The most widely used approach has been serial monitoring of EF by multi-gated acquisition (MUGA) or echocardiogram (ECHO) and limiting the total doxorubicin dose. Biomarkers such as troponin, N-terminal brain natriuretic peptide, and brain natriuretic peptide, or clinical assessment for signs and symptoms of heart failure are also used to assess cardiotoxicity.
Ejection fraction has been shown to be a useful measure of cardiac function in some settings, especially HF. However, HF is a clinical syndrome, while EF is a measure of left ventricular function that is very load dependent; they are not one and the same. For example, a modestly low EF may or may not be associated with HF. A reduction in EF should be viewed as a “biomarker” that is associated with HF or may predict the development of HF. It is, of course, widely used to monitor doxorubicin toxicity in patients receiving doxorubicin, and doxorubicin use is often limited on the basis of EF measurements; however, the use of EF is not without its shortcomings. Despite a number of guidelines for monitoring cardiotoxicity with doxorubicin [25], the evidence for the utility of such recommendations is limited. Monitoring EF adds significant costs [24], and MUGA is associated with radiation exposure as well. In addition, if the results are misleading, a patient may be denied further treatment with a drug for treating their cancer.
In this study a retrospective review of 56 patients followed long-term in one practice who received high doses of PLD, sometimes after previous free doxorubicin exposure, was performed to examine the incidence of doxorubicin-induced HF. Our experience in this study made us aware of the serious limitations of EF monitoring to measure the sequential cardiotoxicity of PLD, and other drugs as well, and has the potential risk of inappropriately altering treatment.
Methods
A retrospective chart review was conducted of patients who received a large cumulative dose of PLD between 1997 and 2017, and were seen by one clinician. In addition, published data from three phase II trials of PLD performed at our institution [18, 19, 26] were also reviewed. Two additional patients who had abnormal EF at diagnosis were also included for illustration. This study was approved by the University of Minnesota Institutional Review Board. As most patients were not on a study, the timing of cardiac function tests was not standardized, as it was part of routine clinical care. Some patients received free doxorubicin, as indicated, before receiving PLD. Most determinations of EF were obtained by MUGA, although in some cases echocardiography (ECHO) was used, as indicated in the Supplementary Table.
Results
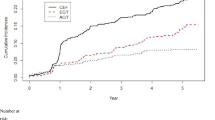
Fifty-six patients who received >450 mg/m2 of doxorubicin (free doxorubicin combined with PLD) were reviewed. Of these, 49 received >500 mg/m2, 28 > 700 mg/m2, 19 > 800 mg/m2, 14 > 1000 mg/m2, and 5 > 1400 mg/m2 (Tables 1; Supplementary Table). There were 32 men and 24 women with a median age at the first doxorubicin dose of 54 (range 15–93). Two other patients (#57 and 58) had an abnormal baseline EF in the absence of symptoms that later normalized. Forty-five patients were followed for >1 year from the start of doxorubicin treatment (range 10–336 months, median 54 months). No follow-up time was available for 10 patients previously reported in earlier studies [18, 19, 26]. In addition, two other patients with existing doxorubicin-induced HF were treated with PLD without clinical deterioration of HF (not shown). The distribution of PLD vs CIVI free doxorubicin vs bolus free doxorubicin is shown in Fig. 1 and Supplementary Table.
Histogram of doxorubicin dose by formulation and type of administration (PLD, solid; civi, shaded; bolus, open). For patient #19 the minimum amount of free doxorubicin is shown (exact amount unknown). For patient #34 the minimum amount of PLD is shown (exact amount of unknown). For patient #53 the minimum amount of PLD is shown (exact amount of unknown)
Two findings are noteworthy. Clinical HF clearly related to doxorubicin was not observed, yet seemingly random changes in sequential measurements of EF were frequently noted, not surprisingly (Fig. 2; Supplementary Table). Within the “normal range” of EF (50–78% for men and 50–87% for women), the measurement varied greatly over time in patients in the absence of symptoms or signs of heart disease (Fig. 2). This is not unexpected and well known to cardiologists, as loading conditions and heart rate can change minute-to-minute, and change left ventricular performance independent of any change in intrinsic contractility. In 17 patients the EF changed by ≥10%, or became abnormal. The EF decreased by >10% within the normal range in 6 patients, and the EF increased by >10% (in 1 by >20%) within the normal range in five patients. The EF became abnormal in five patients, all of whom had a later EF that was in the normal range (patients 10, 15, 16, 32, 34); in one it later became abnormal again (#16). In three other patients the EF was abnormal at the start but rapidly normalized in two (Supplementary Table, patients #57 and 58) and remained abnormal without symptoms in 1 (#24). No patient discontinued PLD due to a change in EF. Most of the EFs in the current study were determined by MUGA (Supplementary Table). It should be noted that a reduction in EF related to chemotherapy may later improve, and then become reduced remote from the time of chemotherapy.
ACE inhibitors and beta-blockers may have a protective effect against the development of HF [27, 28], although this has not been shown in a randomized trial. We therefore determined whether patients were seen by a cardiologist or treated with these agents (Table 1). Six patients were treated at some point with one of these agents, 31 were not, and data were not available for 18 cases.
Discussion
In this study a retrospective review of patients who received ≥450 mg/m2 doxorubicin as PLD and/or free doxorubicin treated in one practice was performed to examine the incidence of doxorubicin-induced HF. In the current study no clear doxorubicin-induced HF was seen in 56 patients who received >450 mg/m2 total doxorubicin dose. Of these, 49 received >500 mg/m2, 28 > 700 mg/m2, 19 > 800 mg/m2, and 14 > 1000 mg/m2. Two patients with pre-existing doxorubicin-induced HF were also treated with PLD without adverse effects (not shown here). An additional observation was the prominent variability in the measured EF within patients in the absence of any clinical signs or symptoms of heart disease. These random changes in EF over time are not unexpected, and may be related to changing cardiac loading conditions (Table 2).
These findings are in marked contrast to earlier reports using bolus administration of free doxorubicin. When given by bolus administration the incidence of doxorubicin-induced HF is dose-related, and has been reported to increase from ~7% at 550 mg/m2 to ~35% at 700 mg/m2 to ~50% at 1000 mg/m2 [2, 29]. When combined with previous reports [18, 19, 21,22,23,24, 29] of patients receiving high doses of PLD, >140 patients have been treated with >500 mg/m2 PLD or PLD combined with free doxorubicin without the development of PLD-related HF.
Many of the cases described here are notable for the long follow-up after doxorubicin exposure. However, much longer-term effects would not be detected with the current study even though some patients were followed for >10 years. In one large study ~98% of cases of “cardiotoxicity” identified by EF occurred within 1 year of completion of chemotherapy [28]. The range of time between the last dose of doxorubicin and the onset of doxorubicin-induced HF is broad, ranging from days to years [1, 15, 30, 31]. Some pediatric and adult cancer survivors have been found to develop cardiomyopathy years after treatment with doxorubicin [30,31,32,33,34,35]. Some animal models also demonstrate progressive doxorubicin-induced cardiomyopathy, and a similar effect is seen with PLD [15]. In addition, genetic variants appear to correlate with the development of doxorubicin-induced cardiotoxicity in children [31]. The findings of the current study may not apply to children.
Several approaches have been taken to try to reduce anthracycline cardiotoxicity, including the use of angiotensin converting enzyme inhibitors and beta-blockers [27, 28], a heavy metal chelator [36], and anti-inflammatory drugs [37]. The use of dexrazoxane from the start of bolus doxorubicin may reduce long-term cardiotoxicity; none of the patients in this study received dexrazoxane. The peak serum level of doxorubicin is important in the induction of cardiotoxicity, and the administration of doxorubicin by CIVI over longer times has been shown to reduce cardiotoxicity in adults [1, 4,5,6,7,8,9,10]. PLD leaves the vascular space at a much slower rate than free doxorubicin, and many studies have reported less cardiotoxicity with PLD than with free doxorubicin [14,15,16,17,18,19,20,21,22,23,24, 29]. Reduced risk of cardiotoxicity is not equal with all liposomal formulations [38]; thus, the conclusions of studies using PLD may not apply to other liposomal doxorubicin formulations that have different stability properties.
Despite the wide use of EF to monitor doxorubicin cardiotoxicity, and the existence of a variety of guidelines and recommendations, the utility of serial EF measurements to predict doxorubicin-induced HF is unclear. This is in part due to the fact that a low EF and heart failure are not the same. One is a measurement, and the other is a clinical syndrome; they don’t have to co-exist. Patients may also have HF with a preserved EF. EF may change modestly from day-to-day in normal subjects, depending on hydration status, similar to blood pressure and heart rate; this is also true in patients being treated with doxorubicin. In addition to the normal day-to-day variation in EF, there is inter- and intra-observer variation in the reading of EF by ECHO. This is especially true when the EF is simply “estimated” by the echocardiographer without objective measurements. It is generally preferred that the ECHO EF be calculated, not estimated, as this tends to lower the degree of intra- and inter-observer variation. When MUGA is used, the EF is always calculated mathematically, so the measurement is considered more precise. Most of the EFs in the current study were determined by MUGA (Supplementary Table).
In addition to physiologic variation, the measurement of EF may not be accurate. Although EF determined by 3-dimensional echocardiography can vary by ~0.05–0.06 (absolute change in EF) due to physiologic changes or test reproducibility [39], with 2-dimensional echocardiography this variability can be ~0.10–0.13 [39], with ~11% being the smallest change in EF that can be recognized with 95% confidence [40]. Although MUGA scans are more accurate, there is still variability in the EF measured by this technique. In one study the 95% CI was −5.4 to +6.4 for inter-observer variability and −4 to +3.5 for intra-observer variability [41]. In a related study, the inter-study variability on patients who were scanned twice on CZT-SPECT cameras had 95% CI of −5.4 to +6.4 [42]. Thus, the limitations of EF measurement must be considered in the interpretation of EF changes. As shown in this series of 53 cases, the EF can vary prominently over time in the absence of cardiac symptoms. It is noteworthy that the early angiographic determination of the EF in normal subjects was limited to <10 subjects.
In the absence of clinical symptoms, the definition of cardiotoxicity is not exact. Some definitions of cardiotoxicity have utilized a decrease in EF of ≥5 to <55% with symptoms of HF, or a decrease in EF of ≥10 to <55% in asymptomatic patients, though other definitions are also used [39, 43, 44]. However, as discussed above, EF varies somewhat even throughout the day in normal people, and in the absence of clinical symptoms, the utility of EF changes to predict doxorubicin-induced HF is unproven.
An analysis of three phase 3 studies found that the left ventricular EF was not an accurate predictor of the development of doxorubicin-induced HF [2]. A study of 20 patients with EF <50% undergoing bone marrow transplant found no difference in survival as compared with 288 patients with preserved EF [45]. A similar finding was seen in a study of 49 patients with EF <50% as compared with 49 controls [46]. The lack of clear benefit of EF monitoring could be due to several factors: intrinsic variability of measuring EF at a given time, random variability of EF over time in normal and abnormal hearts within patients, variation in intravascular volume status when EF is measured, and when the EF is determined in relation to the last dose of the cardiotoxic agent. Further confounding factors include: when and to what degree cardiotoxicity will develop in relation to when and to what degree EF changes occur, and when will cardiotoxicity develop following exposure to a cardiotoxic agent. Finally, if some degree of cardiotoxicity is noted at one time point after cardiotoxin exposure, what is the natural history of cardiac function in the absence and presence of additional cardiotoxin exposure? That is, will changing treatment alter further change in EF, and to what degree does a change in EF alter clinical outcome.
This study is subject to the usual limits of a retrospective study. In addition, as described above, the diagnosis of HF is subjective, and the physicians treating the patients in this study might differ from others in their use of the term. However, the doses received and long-term follow-up are noteworthy. In addition, the observed changes in EF within patients over time, and the range of time over which doxorubicin-induced cardiotoxicity can be observed (days to years), along with the variable time between drug exposure and EF determination, suggest that the routine use of measurements of left ventricular EF to direct doxorubicin use is open to question and needs further study before it can be accepted as a useful approach. An attractive study would be a randomized trial to examine whether monitoring EF can truly predict later significant doxorubicin cardiotoxicity, and whether the medical treatment of asymptomatic EF changes impacts later functional outcome.
This report provides further evidence for the low risk of cardiotoxicity with PLD and CIVI doxorubicin. In addition, the “normal” fluctuations in EFs observed over time, and the potential for cardiotoxicity developing long after doxorubicin exposure, raise the question of the utility of routine cardiac monitoring during doxorubicin administration. While cardiologists are familiar with the limitations of the use of EF, many oncologists may not be. In addition such monitoring adds to the cost of health care. The Medicare fee rates for an ECHO or MUGA scan are in the range of ~$365–890 and $840–2300, respectively [24]. Given the reduction in doxorubicin-induced HF when doxorubicin is given by CIVI or as PLD, and the limited predictive value of EF in predicting doxorubicin cardiotoxicity, the monitoring of EF during treatment is not of proven value. Indeed, as has been suggested, given the prognosis of many patients receiving these drugs, erroneous interpretation of EF changes might inappropriately limit their access to a useful drug [21, 24]. This question is also relevant to the use of EF to monitor cardiotoxicity in other cancer drugs [35, 47], which are also widely used, where the increased cost and true utility are also important. A number of other approaches to predicting doxorubicin-induced cardiomyopathy are under study, although the relationship of early test results with long-term effects will require detailed follow-up [48, 49]. For example, in a small study, the sensitivity to doxorubicin of cardiomyocytes derived from induced stem cells from dermal fibroblasts correlated with the development of doxorubicin-induced HF in the donor [50].
In conclusion, our results question the utility of sequential measurement of EF to guide doxorubicin dosing in the case of adults treated with PLD and also in the setting of doxorubicin given by CIVI. In addition to the expense of testing and the lack of clear benefit in reducing doxorubicin-induced HF, random variation in EF measurement may lead to depriving some patients of a useful treatment. These results may not apply to all liposomal formulations or to children. We agree with Gill et al. and Kushnir et al. [21, 24] that the routine surveillance of EF, at least in adults with PLD in the absence of serious risk factors, does not seem warranted. Even with serious risk factors the utility of cardiac monitoring is seemingly limited.
References
Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M, Muggia FM (1979) Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91(5):710–717
Swain SM, Whaley FS, Ewer MS (2003) Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97(11):2869–2879. doi:10.1002/cncr.11407
Stevenson LW, Perloff JK (1989) The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 261(6):884–888
Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, Valdivieso M, Rasmussen SL, Blumenschein GR, Freireich EJ (1982) Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med 96(2):133–139
Bielack SS, Erttmann R, Winkler K, Landbeck G (1989) Doxorubicin: effect of different schedules on toxicity and anti-tumor efficacy. Eur J Cancer Clin Oncol 25(5):873–882
Lokich J, Bothe A, Zipoli T, Green R, Sonneborn H, Paul S, Philips D (1983) Constant infusion schedule for adriamycin: a phase I-II clinical trial of a 30-day schedule by ambulatory pump delivery system. J Clin Oncol 1(1):24–28
Samuels BL, Vogelzang NJ, Ruane M, Simon MA (1987) Continuous venous infusion of doxorubicin in advanced sarcomas. Cancer Treat Rep 71(10):971–972
Shapira J, Gotfried M, Lishner M, Ravid M (1990) Reduced cardiotoxicity of doxorubicin by a 6-hour infusion regimen. A prospective randomized evaluation. Cancer 65(4):870–873
Hamdan H, Savage PD, Thompson RC, Skubitz KM (1992) A phase I study of ambulatory continuous infusion chemotherapy with cyclophosphamide, doxorubicin, and dacarbazine, (caCAD) for soft tissue sarcomas. J Infus Chemother 2:97–105
Zalupski M, Metch B, Balcerzak S, Fletcher WS, Chapman R, Bonnet JD, Weiss GR, Ryan J, Benjamin RS, Baker LH (1991) Phase III comparison of doxorubicin and dacarbazine given by bolus versus infusion in patients with soft-tissue sarcomas: a Southwest Oncology Group study. J Natl Cancer Inst 83(13):926–932
Alberts DS, Muggia FM, Carmichael J, Winer EP, Jahanzeb M, Venook AP, Skubitz KM, Rivera E, Sparano JA, DiBella NJ, Stewart SJ, Kavanagh JJ, Gabizon AA (2004) Efficacy and safety of liposomal anthracyclines in phase I/II clinical trials. Semin Oncol 31(6 Suppl 13):53–90
Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y (1994) Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res 54(4):987–992
Northfelt DW, Martin FJ, Working P, Volberding PA, Russell J, Newman M, Amantea MA, Kaplan LD (1996) Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS-related Kaposi’s sarcoma. J Clin Pharmacol 36(1):55–63
Working PK, Dayan AD (1996) Pharmacological-toxicological expert report. CAELYX. (Stealth liposomal doxorubicin HCl). Hum Exp Toxicol 15(9):751–785
Working PK, Newman MS, Sullivan T, Yarrington J (1999) Reduction of the cardiotoxicity of doxorubicin in rabbits and dogs by encapsulation in long-circulating, pegylated liposomes. J Pharmacol Exp Ther 289(2):1128–1133
Berry G, Billingham M, Alderman E, Richardson P, Torti F, Lum B, Patek A, Martin FJ (1998) The use of cardiac biopsy to demonstrate reduced cardiotoxicity in AIDS Kaposi’s sarcoma patients treated with pegylated liposomal doxorubicin. Ann Oncol 9(7):711–716
O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C, Group CBCS (2004) Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 15(3):440–449
Skubitz KM (2002) Phase II trial of pegylated-liposomal doxorubicin (Doxil) in mesothelioma. Cancer Investig 20(5–6):693–699
Skubitz KM (2003) Phase II trial of pegylated-liposomal doxorubicin (Doxil) in sarcoma. Cancer Investig 21(2):167–176
Rayson D, Suter TM, Jackisch C, van der Vegt S, Bermejo B, van den Bosch J, Vivanco GL, van Gent AM, Wildiers H, Torres A, Provencher L, Temizkan M, Chirgwin J, Canon JL, Ferrandina G, Srinivasan S, Zhang L, Richel DJ (2012) Cardiac safety of adjuvant pegylated liposomal doxorubicin with concurrent trastuzumab: a randomized phase II trial. Ann Oncol 23(7):1780–1788. doi:10.1093/annonc/mdr519
Gill SE, Savage K, Wysham WZ, Blackhurst DW, Winter WE, Puls LE (2013) Continuing routine cardiac surveillance in long-term use of pegylated liposomal doxorubicin: is it necessary? Gynecol Oncol 129(3):544–547. doi:10.1016/j.ygyno.2013.03.012
Kesterson JP, Odunsi K, Lele S (2010) High cumulative doses of pegylated liposomal doxorubicin are not associated with cardiac toxicity in patients with gynecologic malignancies. Chemotherapy 56(2):108–111. doi:10.1159/000312644
Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A (2000) Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol 11(8):1029–1033
Kushnir CL, Angarita AM, Havrilesky LJ, Thompson S, Spahlinger D, Sinno AK, Tanner EJ, Secord AA, Roche KL, Stone RL, Fader AN (2015) Selective cardiac surveillance in patients with gynecologic cancer undergoing treatment with pegylated liposomal doxorubicin (PLD). Gynecol Oncol. doi:10.1016/j.ygyno.2015.02.020
Brana I, Tabernero J (2010) Cardiotoxicity. Ann Oncol 21(Suppl 7):vii173–vii179. doi:10.1093/annonc/mdq295
Skubitz KM (2002) Phase II trial of pegylated-liposomal doxorubicin (Doxil) in renal cell cancer. Investig New Drugs 20(1):101–104
Bosch X, Rovira M, Sitges M, Domenech A, Ortiz-Perez JT, de Caralt TM, Morales-Ruiz M, Perea RJ, Monzo M, Esteve J (2013) Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 61(23):2355–2362. doi:10.1016/j.jacc.2013.02.072
Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM (2015) Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 131(22):1981–1988. doi:10.1161/CIRCULATIONAHA.114.013777
Uyar D, Kulp B, Peterson G, Zanotti K, Markman M, Belinson J (2004) Cardiac safety profile of prolonged (> or =6 cycles) pegylated liposomal doxorubicin administration in patients with gynecologic malignancies. Gynecol Oncol 94(1):147–151. doi:10.1016/j.ygyno.2004.03.024
Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL, Leisenring WM (2009) Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339:b4606. doi:10.1136/bmj.b4606
Visscher H, Ross CJ, Rassekh SR, Barhdadi A, Dube MP, Al-Saloos H, Sandor GS, Caron HN, van Dalen EC, Kremer LC, van der Pal HJ, Brown AM, Rogers PC, Phillips MS, Rieder MJ, Carleton BC, Hayden MR, Canadian Pharmacogenomics Network for Drug Safety C (2012) Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol 30(13):1422–1428. doi:10.1200/JCO.2010.34.3467
Altena R, Perik PJ, van Veldhuisen DJ, de Vries EG, Gietema JA (2009) Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol 10(4):391–399. doi:10.1016/S1470-2045(09)70042-7
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y, Early Breast Cancer Trialists’ Collaborative G (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366(9503):2087–2106. doi:10.1016/S0140-6736(05)67887-7
Ng R, Better N, Green MD (2006) Anticancer agents and cardiotoxicity. Semin Oncol 33(1):2–14. doi:10.1053/j.seminoncol.2005.11.001
Thavendiranathan P, Abdel-Qadir H, Fischer HD, Camacho X, Amir E, Austin PC, Lee DS (2016) Breast cancer therapy-related cardiac dysfunction in adult women treated in routine clinical practice: a population-based cohort study. J Clin Oncol. doi:10.1200/JCO.2015.65.1505
Asselin BL, Devidas M, Chen L, Franco VI, Pullen J, Borowitz MJ, Hutchison RE, Ravindranath Y, Armenian SH, Camitta BM, Lipshultz SE (2015) Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-hodgkin lymphoma: a report of the Children’s Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol. doi:10.1200/JCO.2015.60.8851
Galal A, El-Bakly WM, Al Haleem EN, El-Demerdash E (2015) Selective A adenosine receptor agonist protects against doxorubicin-induced cardiotoxicity. Cancer Chemother Pharmacol. doi:10.1007/s00280-015-2937-y
Shapiro CL, Ervin T, Welles L, Azarnia N, Keating J, Hayes DF (1999) Phase II trial of high-dose liposome-encapsulated doxorubicin with granulocyte colony-stimulating factor in metastatic breast cancer. TLC D-99 Study Group. J Clin Oncol 17(5):1435–1441
Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH (2013) Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 61(1):77–84. doi:10.1016/j.jacc.2012.09.035
Otterstad JE (2002) Measuring left ventricular volume and ejection fraction with the biplane Simpson’s method. Heart 88(6):559–560
Jensen MM, Schmidt U, Huang C, Zerahn B (2014) Gated tomographic radionuclide angiography using cadmium-zinc-telluride detector gamma camera; comparison to traditional gamma cameras. J Nucl Cardiol 21(2):384–396. doi:10.1007/s12350-013-9844-6
Jensen MM, Haase C, Zerahn B (2015) Interstudy repeatability of left and right ventricular volume estimations by serial-gated tomographic radionuclide angiographies using a cadmium-zinc-telluride detector gamma camera. Clin Physiol Funct Imaging 35(6):418–424. doi:10.1111/cpf.12178
Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F, Group EGW (2012) Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol 23(Suppl 7):vii155–vii166. doi:10.1093/annonc/mds293
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P (2014) Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 15(10):1063–1093. doi:10.1093/ehjci/jeu192
Tang WH, Thomas S, Kalaycio M, Sobecks R, Andresen S, Jarvis J, Rybicki L, Pohlman B, Francis GS, Bolwell BJ (2004) Clinical outcomes of patients with impaired left ventricular ejection fraction undergoing autologous bone marrow transplantation: can we safely transplant patients with impaired ejection fraction? Bone Marrow Transpl 34(7):603–607. doi:10.1038/sj.bmt.1704610
Hurley P, Konety S, Cao Q, Weisdorf D, Blaes A (2015) Hematopoietic stem cell transplantation in patients with systolic dysfunction: can it be done? Biol Blood Marrow Transpl 21(2):300–304. doi:10.1016/j.bbmt.2014.10.011
Chavez-MacGregor M, Niu J, Zhang N, Elting LS, Smith BD, Banchs J, Hortobagyi GN, Giordano SH (2015) Cardiac monitoring during adjuvant trastuzumab-based chemotherapy among older patients with breast cancer. J Clin Oncol 33(19):2176–2183. doi:10.1200/JCO.2014.58.9465
Burdick J, Berridge B, Coatney R (2015) Strain echocardiography combined with pharmacological stress test for early detection of anthracycline induced cardiomyopathy. J Pharmacol Toxicol Methods 73:15–20. doi:10.1016/j.vascn.2015.02.004
Christian JB, Finkle JK, Ky B, Douglas PS, Gutstein DE, Hockings PD, Lainee P, Lenihan DJ, Mason JW, Sager PT, Todaro TG, Hicks KA, Kane RC, Ko HS, Lindenfeld J, Michelson EL, Milligan J, Munley JY, Raichlen JS, Shahlaee A, Strnadova C, Ye B, Turner JR (2012) Cardiac imaging approaches to evaluate drug-induced myocardial dysfunction. Am Heart J 164(6):846–855. doi:10.1016/j.ahj.2012.09.001
Burridge PW, Li YF, Matsa E, Wu H, Ong SG, Sharma A, Holmstrom A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC (2016) Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 22(5):547–556. doi:10.1038/nm.4087
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received no funding.
Conflict of interest
Dr. Skubitz has received research funding from Johnson & Johnson or its subsidiaries and has owned publicly traded stock in JNJ in the past. The other authors have no potential conflict.
Ethical standards
This study was approved by the University of Minnesota IRB and was performed in accordance with the ethical standards of the institutional IRB and the 1964 Helsinki declaration and its later amendments.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was not obtained, per the approval of the University of Minnesota IRB, as it was a retrospective study, no identifying information is presented, and many of the patients are no longer living.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Skubitz, K.M., Blaes, A.H., Konety, S.H. et al. Cardiac safety profile of patients receiving high cumulative doses of pegylated-liposomal doxorubicin: use of left ventricular ejection fraction is of unproven value. Cancer Chemother Pharmacol 80, 787–798 (2017). https://doi.org/10.1007/s00280-017-3420-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3420-8