Abstract
Purpose
To gain a better understanding of the impact of postprogression survival (PPS) and post-trial anticancer therapy on overall survival (OS) in first-line pancreatic cancer patients.
Methods
A literature search identified 54 randomized trials, focusing on gemcitabine monotherapy to eliminate effects of heterogeneity of first-line regimens. We evaluated the relation between OS and either progression-free survival (PFS) or PPS. We also examined whether any association might be affected by the year of completion of trial enrollment.
Results
For all 54 trials, PPS was strongly associated with OS (r = 0.844), whereas PFS was moderately associated with OS (r = 0.623). Average OS and PPS were significantly longer in recent trials than in older trials, (7.29 versus 6.15 months, p < 0.001) and (3.64 versus 2.86 months, p < 0.001), respectively. The correlation between OS and PPS in recent trials was much stronger than that in older trials (r = 0.846 versus 0.729). The relation between OS and PFS in recent and older trials did not differ (r = 0.595 versus 0.563). The percentage of patients with post-trial treatment was significantly higher in recent trials than in older trials (52.7 versus 39.7%, p < 0.001). The rate of post-trial anticancer therapy was significantly associated with OS (r = 0.910).
Conclusions
We found an increase in median PPS in accordance with an increase in median OS in recent trials compared with older trials and that rate of post-trial anticancer therapy was strongly associated with median OS. It is important that researchers be aware of these findings in designing clinical trials of first-line chemotherapy for pancreatic cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer (PC) is the fourth cause of cancer-related death in Europe and the United States [1]. Gemcitabine (GEM) monotherapy has been the standard first-line chemotherapy for patients with unresectable locally advanced or metastatic PC since 1997 [2]. Although various GEM-based combination regimens and GEM-free regimens have been evaluated, only nab-paclitaxel or erlotinib added to GEM or fluorouracil/leucovorin plus irinotecan plus oxaliplatin (FOLFIRINOX) showed a survival benefit over GEM alone in phase III studies [3–5]. The median overall survival (OS) for patients with metastatic disease is less than 12 months, even when patients are treated with the most active chemotherapy regimens [4, 5].
Recent phase III studies CONKO-003 and NAPOLI-1 showed survival benefit of second-line treatment with an oxaliplatin-based regimen or a nanoliposomal irinotecan-based regimen in patients with GEM-refractory PC [6]. In view of the growing number of available drugs, any effect of first-line chemotherapy on OS might be confounded by several lines of subsequent therapy. Indeed, an improvement in progression-free survival (PFS) did not necessarily result in improved OS in a recent randomized phase III MAESTRO trial of evofosfamide in combination with GEM. The GEM arm performed better than the initial assumptions and the receipt of second-line therapy following disease progression was increased in the GEM arm compared with the experimental arm. In a phase III trial comparing GEM plus S-1 with GEM alone, S-1 failed to improved OS when added to GEM and second-line chemotherapy mainly with S-1 in the GEM group may be one reason for this discrepancy [7].
The duration of survival after disease progression (postprogression survival, PPS) and its association with OS have recently been investigated in advanced breast, colorectal, non-small cell lung and gastric cancers [8–11]. However, little is known about PPS in PC. First line chemotherapy regimens investigated in these meta-analysis of PPS were heterogeneous. GEM monotherapy had been the standard first-line chemotherapy for patients with unresectable PC for a long time and the effect of therapy after disease progression on survival in clinical trials of first-line GEM monotherapy is thus of interest. In the present study, we partitioned OS in randomized phase II and III trials for chemotherapy-naïve patients with advanced PC treated with GEM alone into PFS and PPS and assessed the association of each with OS.
Materials and methods
Search strategy and selection of trials
An independent review of Web of Science and Medline citations from 1 January 1997 to 31 April 2015 was carried out. Key words included in the search were ‘pancreatic cancer’, ‘randomized’ and ‘chemotherapy’. The search was limited to randomized controlled phase II and III trials with articles published in English. We reviewed each publication, and selected randomized studies comparing two or more first-line systemic chemotherapeutic agents (including treatment with molecular targeted agents) for unresectable locally advanced or metastatic PC. To find any additional trials, we also searched unpublished data and abstracts from annual meetings of the American Society of Clinical Oncology (from 1997 to 2014) and the European Cancer Conference and European Society of Medical Oncology (from 1997 to 2014). We focused on PPS in patients who received first-line GEM monotherapy, and included randomized trials comparing GEM alone with other regimens for patients with histologically confirmed PC. Trials were eligible if they provided data for both OS and either PFS or time to progression (TTP), whether or not these parameters were explicitly defined. Exclusion criteria included trials designed to assess combined-modality therapy including radiation therapy or surgery (adjuvant or neoadjuvant chemotherapy), and those in which patients had previously been treated with chemotherapy. Two investigators (A.K. and Y.H.) independently abstracted the data from the trials to avoid bias.
We conducted a meta-analysis according to PRISMA. We did not use individual data but published data. The value of information has already been widely utilized in research and generally available. So we further confirm that any aspect of the work covered in this manuscript has been conducted with the ethical approval.
Data abstraction
We analyzed in detail the primary and secondary efficacy end points, following the definitions of the authors of each trial. When not specifically stated by the authors, we considered the primary endpoint to be used for calculation of sample size. For the sake of simplicity, two endpoints (PFS and TTP) based on tumor assessment are collectively referred to as PFS in the present study, similar to the approach adopted in recent reports [8, 10, 11]. Median OS and median PFS were extracted from all trials that provided data for the GEM alone group. Median PPS was defined as median OS minus median PFS for each trial. We also obtained the following information from each report: year of completion of trial enrollment, number of patients in GEM alone group, median age of patients and proportion of patients who received second-line chemotherapy.
Data analysis
We summarized the survival data (median OS, median PFS, median PPS, and median PFS/ median OS) as the average and standard error (SE) for the GEM alone arm. SE was calculated on the basis of previously described models [12]. We also calculated the percentage of OS accounted by PPS for the GEM alone arm as: 100 − (100 × median PFS/median OS). To assess the relation between median OS and either median PFS or median PPS, we used Spearman’s rank correlation coefficient. To account for differences in sample size among trials, we weighed all analyses by the number of patients in the GEM alone arm. In addition, all trials were divided into two groups on the basis of the year in which trial enrolment was completed. Given the number of patients was nearly evenly split with a threshold between 2005 (n = 3329) and 2006 (n = 3527), we dichotomized at year 2005 (older trials, up to and including 2005; recent trials, 2006 and later) to evaluate a possible change in PPS and we assessed whether the evaluated relations might be dependent on the year of completion of trial enrolment. We examined differences in the survival data between older and recent trials by normal approximation of the average survival data (t test). All reported p values correspond to two-sided tests, and those of <0.05 were considered statistically significant. Analyses were carried out with SPSS (version 20.0, SPSS, Chicago, IL, USA) and SAS for Windows release 9.4 (SAS Institute, Cary, NC).
Results
Characteristics of trials
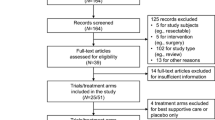
Our research yielded a total of 1648 potentially relevant publications. The selection process for the remaining randomized controlled trials is shown in Fig. 1. A total of 54 trials were finally considered to be highly relevant for the present study (see Supplementary Table 1). The main characteristics of the 54 trials included in the analysis are listed in Table 1. A total of 6856 patients with advanced pancreatic cancer treated with GEM monotherapy were enrolled with a median rate of locally advanced disease of 21% and a median rate of metastatic disease of 79%. Twenty-five of the trials were randomized phase II trials and 29 were phase III trials. Data of post-trial treatment were available in 20 of the trials.
Median OS, PFS, and PPS in all trials and in subgroups based on year of completion of trial enrolment
Among the 54 trials, the average median OS, median PFS and median PPS were 6.73, 3.47 and 3.26 months (Table 2). The average median OS was significantly longer in recent trials than in older trials (7.29 versus 6.15 months, p < 0.001), and this improvement was accompanied by a significant increase in the average median PFS (3.65 versus 3.29 months, p < 0.001) and PPS (3.64 versus 2.86 months, p < 0.001). The average proportion of median OS accounted for by median PFS was significantly larger in recent trials than in older trials (49.61 versus 46.49%, p < 0.001).
Relation between OS and either PFS or PPS
The relation between median OS and either median PFS or median PPS are shown in Figs. 2 and 3, respectively. We found that median PPS was strongly associated with median OS (r = 0.844, p < 0.001) on the basis of Spearman’s correlation coefficient, whereas median PFS was moderately correlated with median OS (r = 0.623, p < 0.001). The association between median OS and median PPS in recent trials (r = 0.846, p < 0.001) was much stronger than that in older trials (r = 0.729, p < 0.001). The correlation between OS and PFS in recent and older trials did not differ (r = 0.595 versus 0.563).
Relation between median overall survival (OS) and median progression-free survival (PFS). a All trials. b Older trials (trial enrollment completed by 2005). c Recent trials (trial enrollment complted in 2006 or later). The area of each circle is proportional to the number of patients in each trial arm. The r values represent Spearman’s rank correlation coefficient
Relation between median overall survival (OS) and median post progression survival (PPS). a All trials. b Older trials (trial enrollment completed by 2005). c Recent trials (trial enrollment completed in 2006 or later). The area of each circle is proportional to the number of patients in each trial arm. The r values represent Spearman’s rank correlation coefficient
Relation between OS and post-trial treatment
Data of post-trial treatment were available in 20 of the 54 trials. Nine of the 20 trials were older trials and the remaining 11 were recent trials. The average median OS was significantly longer in recent trials than in older trials (7.59 versus 6.47 months, p < 0.001), and this improvement was accompanied by a significant increase in average median PPS (3.92 versus 3.08 months, p < 0.001) and average median PFS (3.67 versus 3.39 months, p < 0.001). The percentage of patients with post-study treatment was significantly higher in the recent trials than in the older trials (52.7 versus 39.7%, p < 0.001) (Table 3). The rate of post-trial anticancer therapy was strongly associated with median OS in 20 trials (r = 0.910, p < 0.001) (Fig. 4).
Discussion
The present study examined the duration of PPS in randomized trials in patients with advanced pancreatic cancer treated with GEM monotherapy. Although first line chemotherapy regimens investigated in meta-analysis of postprogression survival in advanced breast, colorectal, non-small cell lung and gastric cancers were heterogeneous, we were able to focus on only patients who received first-line GEM monotherapy because GEM monotherapy had been the standard first-line chemotherapy for patients with unresectable locally advanced or metastatic PC for a long time. We found that median OS was more strongly associated with median PPS than with median PFS. Moreover, we detected an increase in median PPS in accordance with an increase in median OS in recent trials compared with older trials. A similar prolongation of median PPS and median OS has also been described for breast cancer [8], colorectal cancer [9], non-small cell lung cancer [10, 13], and gastric cancer [11]. This is considered to be the result of subsequent active anticancer therapy. Given that the recent phase III studies CONKO-003 and NAPOLI-1 have shown a survival benefit of second-line chemotherapy after failure of GEM [6], increased provision of subsequent potentially active therapy for pancreatic cancers might be expected. In the MAESTRO trial the receipt of second-line therapy following disease progression was increased in the GEM arm compared with the experimental arm particularly for FOLFIRINOX and nab-paclitaxel in combination with GEM. In Japan, clinical trials of S-1 have been conducted since the early 2000s for patients with pancreatic cancer and there were four randomized trials comparing GEM monotherapy with GEM plus S1 [7, 14–16]. Median OS, PFS and PPS of the GEM monotherapy arm in these four trials were 8.0–8.8 months, 3.6–4.1 months and 4.2–5.2 months, respectively. The rate of post-trial anticancer therapy was 58.5–67.0%. The recent increasing number of active compounds used after failure of GEM monotherapy are likely to prolong median PPS as well as OS recently.
Although we focused only on patients who received first-line GEM monotherapy, PFS was significantly longer in recent trials than in older trials. Although the correlation between OS and PFS in recent and older trials did not differ, screening programs may have potential biases. In lead-time bias, earlier detection of tumors via screening may appear to result in longer survival than that in subjects identified by clinical symptoms [17]. Earlier diagnosis of cancer is increasingly acknowledged as a key element of the drive to improve cancer outcomes, and the National Awareness and Early Diagnosis Initiative in UK is addressing this challenge and similar objectives are being pursued by a variety of national initiatives [18–20]. In UK, analysis of diagnostic intervals defined as the duration from the first occurrence of a symptom to the date of cancer diagnosis between the 2001–2002 and 2007–2008 cohorts in pancreatic cancer showed that there was a significant reduction in the interval of 12.6 days (0.42 months) [21]. In our study median PFS was 0.36 months longer in recent trials than in older trials. Lead to bias may be one reason for this prolongation of median PFS.
The present study has several limitations. First, our analysis was based on abstracted data. The use of individual patient data might be expected to allow better characterization of the relation between OS and other end points based on tumor assessment. However, such an approach would restrict the analysis to a small number of trials and would hinder its replication by independent researchers. Second, the results of our study potentially have several confounders due to selection of many heterogeneous trials for analysis. We focused on patients treated with GEM monotherapy to minimize the heterogeneity. The results are generally unaccountable without appropriate adjustment for patient characteristics dependent on differences in predefined eligibility criteria for enrollment in the clinical trials. Third two endpoints (PFS and TTP) based on tumor assessment are considered as the same parameter, following the example of previous reports for advanced breast, non-small cell lung and gastric cancers [8, 10, 11]. TTP is the same as PFS if death does not occur during treatment. However, we also separately analyzed clinical trials providing PFS (n = 40 trials) or TTP (n = 14 trials), and found a consistent association between OS and PPS (data not shown). These data thus support our approach in which these two endpoints are collectively referred to as PFS in the present analysis. Finally, the clinical impact of PPS on OS in PC might be obscure because the duration of OS in patients with PC is much shorter than that in patients with advanced breast, colorectal, non-small cell lung and gastric cancers. Moore and colleagues reported a statistically significant OS benefit of 0.33 months for erlotinib added to GEM [3]. So significant increase in OS in the interval of one month between recent trials and older trials in this study suggest clinical value of PPS with the increasing number of available drugs. As PPS increases, OS can become skewed, and a statistically significant benefit in terms of PFS will likely become masked with OS as the endpoint [22].
In conclusions, there was an apparent increase in median PPS accompanying an increase in median OS in recent trials compared with older trials for patients with advanced PC treated with GEM monotherapy. The rate of post-trial anticancer therapy was strongly associated with median OS and it was significantly higher in recent trials than in older trials. Although OS is the gold standard for efficacy evaluation in phase III trials for advanced PC, it is important that researchers be aware of these findings in designing clinical trials of first-line chemotherapy for patients with advanced PC.
Abbreviations
- PC:
-
Pancreatic cancer
- GEM:
-
Gemcitabine
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PPS:
-
Postprogression survival
- TTP:
-
Time to progression
- SE:
-
Standard error
References
Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E (2013) European cancer mortality predictions for the year 2013. Ann Oncol Off J Eur Soc Med Oncol/ESMO 24(3):792–800. doi:10.1093/annonc/mdt010
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol Off J Am Soc Clin Oncol 15(6):2403–2413
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol Off J Am Soc Clin Oncol 25(15):1960–1966. doi:10.1200/jco.2006.07.9525
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825. doi:10.1056/NEJMoa1011923
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369(18):1691–1703. doi:10.1056/NEJMoa1304369
Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, Gorner M, Molle M, Greten TF, Lakner V, Bischoff S, Sinn M, Dorken B, Pelzer U (2014) Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol Off J Am Soc Clin Oncol 32(23):2423–2429. doi:10.1200/jco.2013.53.6995
Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, Shimamura T, Sho M, Kitano M, Cheng AL, Mizumoto K, Chen JS, Furuse J, Funakoshi A, Hatori T, Yamaguchi T, Egawa S, Sato A, Ohashi Y, Okusaka T, Tanaka M (2013) Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol Off J Am Soc Clin Oncol 31(13):1640–1648. doi:10.1200/jco.2012.43.3680
Saad ED, Katz A, Buyse M (2010) Overall survival and post-progression survival in advanced breast cancer: a review of recent randomized clinical trials. J Clin Oncol Off J Am Soc Clin Oncol 28(11):1958–1962. doi:10.1200/jco.2009.25.5414
Petrelli F, Barni S (2013) Correlation of progression-free and post-progression survival with overall survival in advanced colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol/ESMO 24(1):186–192. doi:10.1093/annonc/mds289
Hayashi H, Okamoto I, Morita S, Taguri M, Nakagawa K (2012) Postprogression survival for first-line chemotherapy of patients with advanced non-small-cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol/ESMO 23(6):1537–1541. doi:10.1093/annonc/mdr487
Kawakami H, Okamoto I, Hayashi H, Taguri M, Morita S, Nakagawa K (2013) Postprogression survival for first-line chemotherapy in patients with advanced gastric cancer. Eur J Cancer (Oxford, England: 1990) 49 (14):3003–3009. doi:10.1016/j.ejca.2013.05.022
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled clinical trials 7(3):177–188
Hotta K, Kiura K, Fujiwara Y, Takigawa N, Hisamoto A, Ichihara E, Tabata M, Tanimoto M (2011) Role of survival post-progression in phase III trials of systemic chemotherapy in advanced non-small-cell lung cancer: a systematic review. PLoS One 6(11):e26646. doi:10.1371/journal.pone.0026646
Nakai Y, Isayama H, Sasaki T, Sasahira N, Tsujino T, Toda N, Kogure H, Matsubara S, Ito Y, Togawa O, Arizumi T, Hirano K, Tada M, Omata M, Koike K (2012) A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer 106(12):1934–1939. doi:10.1038/bjc.2012.183
Ozaka M, Matsumura Y, Ishii H, Omuro Y, Itoi T, Mouri H, Hanada K, Kimura Y, Maetani I, Okabe Y, Tani M, Ikeda T, Hijioka S, Watanabe R, Ohoka S, Hirose Y, Suyama M, Egawa N, Sofuni A, Ikari T, Nakajima T (2012) Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC-01 study). Cancer Chemother Pharmacol 69(5):1197–1204. doi:10.1007/s00280-012-1822-1
Sudo K, Ishihara T, Hirata N, Ozawa F, Ohshima T, Azemoto R, Shimura K, Nihei T, Nishino T, Nakagawa A, Nakamura K, Hara T, Tada M, Mikata R, Tawada K, Yokosuka O, Nakaji S, Yamaguchi T (2014) Randomized controlled study of gemcitabine plus S-1 combination chemotherapy versus gemcitabine for unresectable pancreatic cancer. Cancer Chemother Pharmacol 73(2):389–396. doi:10.1007/s00280-013-2368-6
Poruk KE, Firpo MA, Adler DG, Mulvihill SJ (2013) Screening for pancreatic cancer: why, how, and who? Ann Surg 257(1):17–26. doi:10.1097/SLA.0b013e31825ffbfb
Neal RD (2009) Do diagnostic delays in cancer matter? Br J Cancer 101(Suppl 2):S9–S12. doi:10.1038/sj.bjc.6605384
Richards MA (2009) The national awareness and early diagnosis initiative in England: assembling the evidence. Br J Cancer 101(Suppl 2):S1–S4. doi:10.1038/sj.bjc.6605382
Olesen F, Hansen RP, Vedsted P (2009) Delay in diagnosis: the experience in Denmark. Br J Cancer 101(Suppl 2):S5–S8. doi:10.1038/sj.bjc.6605383
Neal RD, Din NU, Hamilton W, Ukoumunne OC, Carter B, Stapley S, Rubin G (2014) Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br J Cancer 110(3):584–592. doi:10.1038/bjc.2013.791
Broglio KR, Berry DA (2009) Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 101(23):1642–1649. doi:10.1093/jnci/djp369
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kasuga, A., Hamamoto, Y., Takeuchi, A. et al. Positive relationship between subsequent chemotherapy and overall survival in pancreatic cancer: meta-analysis of postprogression survival for first-line chemotherapy. Cancer Chemother Pharmacol 79, 595–602 (2017). https://doi.org/10.1007/s00280-017-3263-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3263-3