Abstract
Background
Very little is known about the pharmacokinetics of chemotherapeutic agents in patients also being treated with continuous ambulatory peritoneal dialysis. We sought to evaluate the pharmacokinetics of cisplatin and 5-fluorouracil in plasma and peritoneal dialysate in a patient being treated for esophageal adenocarcinoma.
Methods
A single patient with esophageal adenocarcinoma and on peritoneal dialysis for end-stage renal disease was treated with cisplatin 25 mg/m2 on day 1 of weeks 1 and 5 and continuous infusional 5-fluorouracil 1000 mg/m2/day on days 1–4 of weeks 1 and 5 along with daily radiation therapy. Intense plasma and dialysate sampling was performed during the week 5 administration, followed by quantitation of platinum by atomic absorption spectrophotometry and 5-fluorouracil by LC–MS/MS.
Results
Following systemic administration, clearance of ultrafilterable (active) platinum over the first 6 h was 20.8 L/h, which is lower than previously reported clearance levels of ultrafilterable platinum. Total platinum AUC was 131 μg h/mL, also higher than an AUC previously reported for total platinum in patients with normal renal function. Platinum-related material was detected in the peritoneal cavity, but this is likely inactive. 5-Fluorouracil penetrated the intraperitoneal cavity, but the contribution of peritoneal dialysis to drug clearance was negligible at 0.072 %.
Conclusions
Administration of intravenous cisplatin and 5-fluorouracil chemotherapy to a patient treated with continuous ambulatory peritoneal dialysis is feasible, but clearance in dialysate is nominal, thus suggesting that dose reduction is indicated for cisplatin. Systemic drug administration results in limited intraperitoneal penetration of 5-fluorouracil and inactive platinum species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of systemic chemotherapy for the treatment of many types of cancer is often complicated by the presence of comorbid conditions. Whereas formal pharmacokinetic studies have guided dose recommendations for several agents in patients with organ dysfunction [1, 2], often chemotherapy dosing recommendations are made based on known elimination pathways in the absence of PK data. In patients with renal dysfunction, cancer chemotherapeutics that have renal excretion are often dose-reduced based on the level of renal failure. Patients with end-stage renal disease who undergo dialysis present a unique challenge, as clearance is episodic at the time of dialysis and may vary by agent and metabolite. The context of peritoneal dialysis is particularly vexing, as clearance will depend on access of the drug to the peritoneal cavity and the efficiency of removal in the dialysate. In this report, we describe the pharmacokinetics of cisplatin and 5-fluorouracil in a patient undergoing peritoneal dialysis.
Cisplatin, a platinating agent, is used as part of multiple treatment regimens for a multitude of malignancies and can be associated with a decrease in renal function, which is the organ also responsible for its clearance. In rare instances, a patient requiring cisplatin chemotherapy may also have end-stage renal disease that is managed by continuous ambulatory peritoneal dialysis (CAPD). Little if anything is known regarding the appropriate dosing of this agent in patients on CAPD with only two case reports identified in the literature [3, 4], one of which involves the treatment of a child where drug pharmacokinetics and pharmacodynamics differ from that of adults. Slightly more data are available in patients receiving cisplatin while on hemodialysis; however, these are mainly case reports, and a wide variety of cisplatin doses, dosing schedules and hemodialysis schedules were used [5–8]. The largest study in hemodialysis patients was a dose–escalation study of cisplatin performed in five patients. These patients were able to tolerate up to full-dose cisplatin for treatment of lung cancer while on hemodialysis with minimal toxicity [9]. Despite these results, it is generally thought that one should exercise caution when using cisplatin in patients with renal failure, and consideration should be given to applying a dose reduction.
The administration of 5-fluorouracil in patients on CAPD is also not well described. Only one case report provides pharmacokinetic data from plasma samples, but we could not identify any studies of how 5-fluorouracil penetrates the intraperitoneal fluid [3]. Cisplatin and 5-fluorouracil are administered together in many treatment regimens necessitating the need for further pharmacokinetic data regarding the administration of these drugs in patients on CAPD. In an effort to better characterize the systemic elimination, peritoneal penetration and associated toxicities of these agents in a patient on CAPD, we conducted a single-patient pharmacokinetic evaluation of cisplatin and 5-fluorouracil in the plasma and peritoneal dialysate of a patient receiving cisplatin and 5-fluorouracil for the treatment of early-stage esophageal adenocarcinoma.
Materials and methods
Patient presentation
A 72-year-old female presented with gastroesophageal reflux, weight loss and dysphagia to solids and liquids. An upper endoscopy revealed the presence of a mass in the distal esophagus with a biopsy confirming a poorly differentiated esophageal adenocarcinoma. Endoscopic ultrasound suggested invasion of the adventitia consistent with a T3 lesion. Staging CT scans showed corresponding thickening of the esophagus but no evidence of metastatic disease, and she was determined to have a stage IIB esophageal adenocarcinoma. Her medical history was complicated by multiple comorbid conditions including coronary artery disease requiring bypass grafting, both thoracic and abdominal aneurysms that had not been surgically corrected, carotid stenosis requiring a carotid endarterectomy, gout and end-stage renal disease secondary to hypertension for which she had been receiving CAPD for the past 2.5 years. Her residual renal function, evaluated by measuring a urine 24-h creatinine clearance at the time of presentation, was 7 mL/min. She had no family history of cancer. She reported a 50 pack-year smoking history and continued to smoke at the time of presentation. She denied alcohol or drug use.
A multidisciplinary evaluation by medical, radiation and surgical oncologists recommended a definitive non-surgical approach with systemic cisplatin, 5-fluorouracil and daily radiation. Standard chemotherapeutic dosing in this setting consists of cisplatin 75 mg/m2 on day 1 of weeks 1, 5, 8 and 11, 5-fluorouracil 1000 mg/m2/day via continuous infusion over the first 96 h of weeks 1, 5, 8 and 11 and 50 Gy of radiation in 25 fractions over the first 5 weeks of therapy [10, 11]. After consulting with her nephrologist, one-third of the standard dose of cisplatin (25 mg/m2 on day 1 of weeks 1, 5, 8 and 11) was recommended due to her underlying renal failure. In the second cycle of therapy (beginning week 5), pharmacokinetic samples were obtained from both plasma and peritoneal dialysate after the patient provided written informed consent to participate in a pharmacokinetic sampling protocol approved by the institutional review board. CAPD was continued throughout her treatment course including during chemotherapy administration and consisted of 4 exchanges of 2000 mL of 2.5 % dextrose peritoneal dialysate solution daily (exchanged every 6 h). Complications incurred over the course of her treatment included hypotension, neutropenia, anemia requiring intermittent transfusion of packed red blood cells, thrombocytopenia, ongoing dysphagia and odynophagia, hypokalemia and hypocalcemia. Upon completion of two cycles of chemotherapy and radiation, her blood pressure improved, blood counts normalized and electrolyte disturbances resolved but difficulty with oral intake of both solids and liquids persisted. Restaging CT scans obtained after the 5-week chemoradiation period showed interval development of metastatic disease to the liver. Given her progressive disease, multiple comorbid conditions and post-treatment functional status, additional chemotherapy was not administered and a decision was made with the patient and her family to pursue a palliative approach. The patient expired from her disease shortly thereafter.
Pharmacokinetic analysis of cisplatin and 5-fluorouracil
Cisplatin chemotherapy was initiated immediately following exchange of peritoneal dialysate. A dose of 43 mg of cisplatin was administered (25 mg/m2 with a body surface area of 1.7 m2) as a 30-min infusion. 5-Fluorouracil infusion was initiated upon completion of cisplatin infusion. A total dose of 6800 mg of 5-fluorouracil was administered (1000 mg/m2/day × 4 days) as a 96-h infusion. Blood samples (4 mL collected in a sodium heparin tube) were obtained at the following time points: predose (prior to cisplatin infusion) and then at 15 min, 30, 60, 65, 75, 90, 120, 180, 240, 300, 420 min, and 24, 48, 72 and 96 h. Additional samples were collected weekly for a total duration of 28 days. Tubes were placed immediately in a wet ice bath and then spun at 1000×g for 5 min at 4 °C. Plasma was aspirated and stored at −70 °C until analysis. Dialysate samples (4 mL collected in a sodium heparin tube) were obtained at the following time points: predose (prior to cisplatin infusion) and at 6, 12, 18, 24, 48, 72 and 96 h, followed by samples collected weekly at outpatient office visits for a total duration of 28 days. All samples were immediately placed in a wet ice bath following collection and then spun at 1000×g for 5 min at 4 °C. Acellular material was aspirated and stored at −70 °C until analysis.
Concentrations of total platinum and ultrafilterable platinum (platinum not bound to macromolecules) were quantitated by atomic absorption spectrophotometry (AAS), and 5-fluorouracil concentrations were quantitated by LC–MS/MS as previously described [12, 13]. Pharmacokinetic parameters were derived by non-compartmental modeling using PK Solutions 2.0 (Summit Research Services, Montrose, CO; www.summitPK.com).
Results
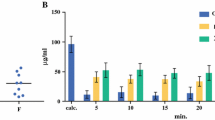
Pharmacokinetic parameters are provided in Table 1, and platinum and 5-fluorouracil plasma and dialysate pharmacokinetic profiles are displayed in Figs. 1 and 2, respectively. Clearance of plasma ultrafilterable platinum within the first 6 h (Cl0–6) was 20.8 L/h. This is in comparison with previously reported plasma unbound platinum clearance levels of 35.5 L/h in patients with normal renal function [14]. Plasma total platinum clearance (Cl0–696) was 0.211 L/h, and plasma ultrafilterable platinum clearance (Cl0–696) was 1.13 L/h. At the end of the 28-day assessment period, only total platinum was still measureable in the plasma, all of which was macromolecule bound and thus inactive. Platinum species penetrate the peritoneal dialysate, with ultrafilterable platinum being near equivalent to total platinum (AUC0–696 of total platinum in dialysate 22.4 μg h/mL and AUC0–696 of ultrafilterable platinum in dialysate 20.9 μg h/mL), and these species were nearly eliminated by the end of the 28-day assessment period. Higher levels of 5-fluorouracil were seen in the plasma as compared to the dialysate—C max 567 vs. 196 μg/mL and AUC0–696 50.0 vs. 12.1 μg h/mL, respectively. The total amount of 5-fluorouracil eliminated by means of dialysis was 0.0049 mg or 0.072 % of the dose.
Discussion
Our results indicate that the exposure to cisplatin in a patient on CAPD was higher than the population average of adults with normal renal function. During and immediately following the cisplatin dose, an acute rise in the plasma concentrations of both total and ultrafilterable platinum was seen where concentrations peaked around 1 h. After the end of infusion, ultrafilterable platinum rapidly decreased with a half-life of 1.26 h, reflecting the highly reactive nature of cisplatin in plasma. Thereafter, platinum concentrations slowly decreased and could be detected out to 28 days, likely reflecting low molecular weight platinum species (ultrafilterable, but likely no longer reactive). The ultrafilterable platinum plasma clearance of 20.8 L/h calculated based on AUC0–6 in our subject was somewhat lower than the 35.5 L/h population average previously reported in patients with normal kidney function [14], resulting in a larger exposure.
The peak peritoneal platinum was observed at our first sampling time of 6 h after the start of infusion. The ultrafilterable platinum and total platinum exposures were almost identical. If the ultrafilterable (low molecular weight) platinum had been reactive, total platinum should have been much higher than ultrafilterable platinum. This suggests that the platinum in the peritoneal cavity is mostly ultrafilterable with a small molecular weight, but is no longer capable of reaction and consequently is inactive. We hypothesize that the small molecular weight, inactive platinum species in plasma equilibrates with that in the peritoneal cavity. This is supported by the observations that total peritoneal platinum concentrations at no point exceed plasma ultrafilterable platinum concentrations and that their concentration versus time profiles parallel each other. Consequently, peritoneal dialysis is not expected to contribute significantly to cisplatin clearance, nor is systemically administered cisplatin expected to contribute much to treatment of peritoneal disease by diffusion into the peritoneal cavity. Any cytotoxic effect of cisplatin on peritoneal disease is likely delivered by plasma exposure of tumor tissue. In the case of our patient, it is therefore most likely that any sustained toxicities were due to a lack in clearance capabilities of active platinum from the systemic circulation as opposed to sustained overexposure to active platinum within the peritoneal dialysate. Administration of a higher dose or more frequent dosing may have resulted in greater toxicity.
It is notable that the systemic administration of cisplatin does not result in significant penetration into the peritoneal cavity. Currently, intraperitoneal cisplatin chemotherapy is indicated for the treatment of ovarian, fallopian tube and primary peritoneal malignancies [15]. Most studies have been conducted in patients with ovarian cancer where three randomized phase III trials have shown survival benefit with a combination of intravenous and intraperitoneal chemotherapy, particularly in patients with optimally debulked intra-abdominal disease [16–18]. In our pharmacokinetic sampling of peritoneal dialysate, we did not observe the presence of active platinum compounds. These findings may in part explain the additional benefit of intraperitoneal cisplatin administration in ovarian cancer patients—tissues are exposed to active platinum compounds through both the systemic and intraperitoneal compartments. Even if these patients were to have some platinum penetration into the peritoneal cavity following intravenous drug administration, it is quite possible that this would be inactive platinum.
Our patient tolerated the toxicities associated with her treatment, and our observed pharmacokinetic results suggest that her ability to clear the drug was relatively low at approximately 59 % of the reported population average. Based on our post hoc assessment of ultrafilterable clearance, the 66 % dose reduction resulted in an exposure that is comparable to patients with normal renal function treated with a regular dose [19].
To our knowledge, the pharmacokinetics of 5-fluorouracil have not been evaluated in the peritoneal dialysate/intra-abdominal compartment following intravenous administration. In our patient, 5-fluorouracil concentrations in the dialysate increase while plasma 5-fluorouracil concentrations remain at a relatively steady state. Dialysate exposure to 5-fluorouracil is approximately 24 % of plasma exposure; however, only 0.072 % of the dose was cleared by means of dialysis, whereas approximately 10 % of 5-fluorouracil is thought to be excreted through the kidney under normal conditions [20]. The average 5-fluorouracil clearance is reported to be 2–3 L/min/m2, compared to the 1.3 L/min/m2 observed in our patient. The lower observed 5-fluorouracil clearance, however, may merely reflect the notoriously high between-subject variability seen with 5-fluorouracil clearance [21].
The fact that this is a report of a single-patient experience limits the generalizability of our results. Our data suggest that patients receiving CAPD should receive approximately 33 % of the standard dose of cisplatin. Elimination of all active platinum species by the end of the treatment cycle suggests that administration of subsequent cycles of cisplatin at the same dose should not cause adverse effects secondary to cisplatin accumulation.
References
Venook AP, Egorin MJ, Rosner GL, Hollis D, Mani S, Hawkins M, Byrd J, Hohl R, Budman D, Meropol NJ, Ratain MJ (2000) Phase I and pharmacokinetic trial of gemcitabine in patients with hepatic or renal dysfunction: cancer and Leukemia Group B 9565. J Clin Oncol 18:2780–2787
Venook AP, Enders Klein C, Fleming G, Hollis D, Leichman CG, Hohl R, Byrd J, Budman D, Villalona M, Marshall J, Rosner GL, Ramirez J, Kastrissios H, Ratain MJ (2003) A phase I and pharmacokinetic study of irinotecan in patients with hepatic or renal dysfunction or with prior pelvic radiation: CALGB 9863. Ann Oncol 14:1783–1790
Okuyama M, Motoyama S, Maruyama K, Ohta H, Tsuchiya N, Oyake J, Ogawa J (2005) Chemotherapy for an esophageal cancer patient undergoing continuous ambulatory peritoneal dialysis for chronic renal failure and measurement of plasma concentration of the drug. Gan To Kagaku Ryoho 32:1029–1032
Sebestyen J, Garg U, Lewing KB, Warady BA, Abdel-Rahman S, Blowey DL (2010) Cisplatin pharmacokinetics in a child receiving peritoneal dialysis. Pediatr Nephrol 25:1185–1189
Tomita M, Kurata H, Aoki Y, Tanaka K, Kazama JJ (2001) Pharmacokinetics of paclitaxel and cisplatin in a hemodialysis patient with recurrent ovarian cancer. Anticancer Drugs 12:485–487
Zahra MA, Taylor A, Mould G, Coles C, Crawford R, Tan LT (2008) Concurrent weekly cisplatin chemotherapy and radiotherapy in a haemodialysis patient with locally advanced cervical cancer. Clin Oncol (R Coll Radiol) 20:6–11
Arai Y, Oyama T, Hotta K, Tomori A, Miyata Y (2008) Successful treatment with 5-fluorouracil and cis-dichlorodiammineplatinum combined with 60 Gy of radiation in a case of advanced esophageal cancer complicated with chronic renal failure treated with hemodialysis. Nihon Shokakibyo Gakkai Zasshi 105:1482–1488
Cho H, Imada T, Masudo K, Doi C, Inaba M, Tokunaga M, Takanashi Y (2000) Combined 5-FU and CDDP in a gastric cancer patient undergoing hemodialysis—pharmacokinetics of 5-FU and CDDP. Gan To Kagaku Ryoho 27:2135–2138
Watanabe R, Takiguchi Y, Moriya T, Oda S, Kurosu K, Tanabe N, Tatsumi K, Nagao K, Kuriyama T (2003) Feasibility of combination chemotherapy with cisplatin and etoposide for haemodialysis patients with lung cancer. Br J Cancer 88:25–30
Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL (1999) Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). JAMA 281:1623–1627
National Comprehensive Cancer Network (NCCN) Clinical practice guidelines in oncology, esophageal and esophagogastric junction cancers version 3. 2015. http://www.nccn.org. Accessed 21 Sept 2015
Kosovec JE, Egorin MJ, Gjurich S, Beumer JH (2008) Quantitation of 5-fluorouracil (5-FU) in human plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 22:224–230
Colville H, Dzadony R, Kemp R, Stewart S, Zeh HJ 3rd, Bartlett DL, Holleran J, Schombert K, Kosovec JE, Egorin MJ, Beumer JH (2010) In vitro circuit stability of 5-fluorouracil and oxaliplatin in support of hyperthermic isolated hepatic perfusion. J Extra Corpor Technol 42:75–79
Urien S, Lokiec F (2004) Population pharmacokinetics of total and unbound plasma cisplatin in adult patients. Br J Clin Pharmacol 57:756–763
Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B (1996) Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 335:1950–1955
Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, Wadler S, Sickel J (2001) Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 19:1001–1007
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LF, Walker JL, Burger RA (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34–43
Cristea M, Han E, Salmon L, Morgan RJ (2010) Practical considerations in ovarian cancer chemotherapy. Ther Adv Med Oncol 2:175–187
Ikeda K, Terashima M, Kawamura H, Takiyama I, Koeda K, Takagane A, Sato N, Ishida K, Iwaya T, Maesawa C, Yoshinari H, Saito K (1998) Pharmacokinetics of cisplatin in combined cisplatin and 5-fluorouracil therapy: a comparative study of three different schedules of cisplatin administration. Jpn J Clin Oncol 28:168–175
Gusella M, Rebeschini M, Cartei G, Ferrazzi E, Ferrari M, Padrini R (2005) Effect of hemodialysis on the metabolic clearance of 5-fluorouracil in a patient with end-stage renal failure. Ther Drug Monit 27:816–818
Grem JL (2000) 5-fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development. Invest New Drugs 18:299–313
Acknowledgments
This project was supported by the Case Comprehensive Cancer Center (P30CA043703), Case Western Reserve University Clinical and Translational Science Collaborative (UL1TR000439) and the University of Pittsburgh Cancer Institute Cancer Pharmacokinetics and Pharmacodynamics Facility (P30CA047904). Dr. Eads is a recipient of a K12 Paul Calabresi Scholar Award (2K12CA076917-17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Eads, J.R., Beumer, J.H., Negrea, L. et al. A pharmacokinetic analysis of cisplatin and 5-fluorouracil in a patient with esophageal cancer on peritoneal dialysis. Cancer Chemother Pharmacol 77, 333–338 (2016). https://doi.org/10.1007/s00280-015-2939-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2939-9