Abstract
Background
Adjuvant chemotherapy is gaining an increasing role in resectable gastric cancer. Customizing chemotherapy on the basis of chemosensitivity may improve outcome, and putative predictive molecular markers have been mostly evaluated in Asian patients. We profiled key DNA and damage signaling factors and correlated them with outcome, in a European cohort.
Methods
Formalin-fixed tumor samples obtained from surgical specimens of patients treated with adjuvant cisplatin-based chemotherapy for gastric cancer were analyzed. Immunohistochemistry (IHC) was performed to analyze excision repair cross-complementing gene 1 (ERCC1) and thymidylate synthase (TS) expression, and p53 mutations were detected with direct sequencing.
Results
Among the 68 patient recruited, the median age was 69 (range 30–74), and UICC stage was III in 44 patients (65 %). With a median follow-up of 40.5 months, disease-free and overall survival were 18.0 (95 % CI 13.4–22.76) and 56 months (95 % CI 44.87–67.13), respectively. ERCC1 score was 0 in 14 out 67 (21 %) cases, 1 in 19 (28 %), 2 in 20 (30 %) and 3 in 14 cases (21 %). Longer overall survival (p = 0.04) was found in patients categorized as ERCC1 negative by IHC according to median score. TS score was 0 in 16 out 67 (24 %) cases, 1 in 27 (40 %), 2 in 16 (24 %) and 3 in 8 cases (12 %). Mutations of p53 were found in 21 out 66 (32 %) cases. Neither TS nor p53 were found to correlate with outcome.
Conclusion
Excision repair cross-complementing gene 1 by IHC might predict patients more likely to benefit from adjuvant cisplatin-based chemotherapy in curatively resected gastric cancer. In patients exhibiting ERCC1 positive tumors, alternative regimens should be evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In gastric cancer, surgery remains the mainstay of cure for patients without distant metastases. In specialized centers with high annual caseloads, D2 dissection is the recommended type of surgery [1].
Perioperative chemotherapy has been implemented in European countries following the results of the MAGIC trial, showing an increase in 5-year overall survival (OS) from 23 to 36 % with the ECF (epirubicin, cisplatin, 5-fluorouracil/5FU) regimen, as opposed to surgery alone [2]. Adjuvant chemoradiation, an alternative strategy often practiced in the US, has demonstrated an OS advantage over surgery alone [3, 4]. Of note, in both strategies, most of the patients underwent suboptimal regional lymphnode dissection.
Despite the abundance of randomized studies, there is limited evidence of efficacy of adjuvant chemotherapy in gastric cancer [5]. Nonetheless, meta-analyses of postoperative chemotherapy reported a small survival benefit [6–10]. Intriguing results of two large randomized trials of adjuvant chemotherapy conducted in Asia were recently published. In the ACTS-GC study, the administration of the oral fluoropyrimidine S1 in stage II/III gastric cancer following D2 gastrectomy increased OS compared with surgery alone [11, 12]. The CLASSIC trial reported an improvement of 15 % in 3-year disease-free survival (DFS) in patients treated with adjuvant oxaliplatin and capecitabine, compared to surgery alone (HR 0.56, p < 0.0001) [13].
The ability to identify patients with increased sensitivity to chemotherapy would allow a better pre-selection based on the individual genetic profile. The excision repair cross-complementing gene 1 (ERCC1) plays an essential role for nucleotide excision repair (NER), and its altered expression has been associated with resistance to platinum compounds. In metastatic gastric cancer, it has been shown that outcome is worse in patients treated with 5FU/cisplatin and exhibiting ERCC1 overexpression [14, 15]. Moreover, thymidylate synthase (TS), a key enzyme involved in the metabolism of 5FU, seems to play a role in resistance to fluoropyrimidines when overexpressed [16, 17], although this finding is controversial [18, 19]. Furthermore, inactivating mutations in p53 gene, encoding for a protein involved in preventing genomic mutations, appear to modulate the cytotoxic effects of 5FU, doxorubicin and cisplatin. Indeed, in several neoplasms including gastric cancer, p53 alteration (either induced by protein overexpression or gene mutations) has been related with resistance to doxorubicin and platinum compounds [20, 21].
We here investigated ERCC1, p53 and TS in a cohort of European patients with curatively resected gastric adenocarcinoma, aiming to identify molecular alterations able to predict the efficacy of adjuvant cisplatin-based chemotherapy.
Patients and methods
Patients’ population
This study is a retrospective analysis of 68 patients treated between December 1996 and October 2009. This study has been approved by the institutional review board of the recruiting centers. A written informed consent was requested.
All patients had undergone curative surgery with D2 dissection for gastric adenocarcinoma, were pathological stage II to IIIB according to the UICC classification and were treated with adjuvant ECF regimen. A chest X-ray and abdomino-pelvic CT scan were routinely performed before surgery, and additional exams were considered only in case of clinical suspicion of distant metastases. In all cases, macroscopic disease was eradicated by surgery, and resection margins were free of tumor at histopathological examination.
The clinical outcome was monitored for each patient from surgery to death or last follow-up.
Treatment and follow-up
Adjuvant chemotherapy consisted in 4–6 courses of ECF, administered as follows: Cisplatin 60 mg/m2 and Epirubicin 50 mg/m2 both on day 1 every 3 weeks, and 5FU 200 mg/m2/day as a protracted continuous infusion. Dose modifications were applied according to the original schedule [22]. Chemotherapy started in all patients within 8 weeks from surgery. Surveillance after completion of adjuvant chemotherapy occurred at 3-month intervals for 2 years; then at 6-month intervals for 3 years; and consisted of physical examination, complete blood count and liver function testing; radiological exams (in general, abdominal ultrasound or CT scan every 6 months for 5 years) and upper endoscopy (annually, or if clinically indicated) were performed according to the policy of each institution.
Molecular analyses
Formalin-fixed, paraffin-embedded (FFPE) tissue was available for immunohistochemical and molecular analyses for all cases. Genomic DNA (obtained from 3 sections 3 μm thick) was extracted from a single representative FFPE tissue block (containing ≥70 % of neoplastic cells) using the QIAamp Mini kit (Qiagen, Chatsworth, CA, USA), according to the manufacturer’s instructions. Published criteria for accurate block and tumor area selection were applied [23]. By comparison, total RNA was also extracted from a paired healthy mucosa sample (Fig. 1).
ERCC1 and TS analysis
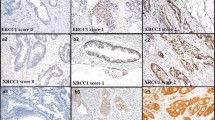
Immunohistochemistry (IHC) was performed on 3-μm-thick tissue section by using anti-ERCC1 (clone 8F1, dilution 1:50; ThermoScientific, Erembodegem, Belgium) and anti-TS (clone TS106, dilution 1:50; Dako, Glostrup, Denmark) monoclonal antibodies. ERCC1 was performed on Ventana BENCHMARK® XT instrument using UltraView DAB kit (Ventana Medical Systems, Tucson, USA), whereas DAKO Autostainer (Dako, Glostrup, Denmark) was used for TS immunostaining. Briefly, for epitope retrieval, slides were exposed on heat EDTA; then, endogenous peroxidase activity was blocked by incubation with 3 % H2O2 (ERCC1: 30 min EDTA and 4 min H2O2—TS: 14 min EDTA and 10 min H2O2). ERCC1 primary antibody incubation was carried out for 32 min at 37 °C, whereas the anti-TS incubation was carried out for 60 min at room temperature. Immunoreaction was revealed by secondary antibody incubation (ERCC1 8 min—TS 30 min) with 3′-3′-diaminobenzidine as the chromogen and Mayer’s hematoxylin as the counterstain. Endothelial cells of normal tonsil tissues and proliferating germinal center lymphocytes were included as positive controls for ERCC1 and TS, as previously suggested [24, 25].
Immunostaining was evaluated under a light microscope by an expert pathologist. For ERCC1, a positive staining was assigned when tumor cells showed nuclear reactivity, while for TS, on the basis of both nuclear and cytoplasmic reactivity. Since to date there are no standardized guidelines for ERCC1 and TS staining evaluation on gastric tumors, an H-score usually utilized in the evaluation of ERCC1 in non-small-cell lung cancer was applied [24]. In detail, the intensity of staining was scored on a scale of 0–3+; 3+ indicating the higher intensity using normal tonsil tissue as positive control. The percentage of positive tumor cells was scored as follows: 0 if 0 %; 0.1 if 1–9 %; 0.5 if 10–49 %; 1 if 50 % or more. Semiquantitative H-score was obtained from intensity multiplied with positive cells, with values ranging from 0 to 3. The median value of all H-score was chosen as the cutoff point to determine positive or negative tissues according the literature [24, 25].
p53 mutational analysis
Since more than 90 % of p53 mutations occur in exons 4–10, the presence of p53 mutations was sought in these regions. All tumor samples were subjected to automated sequencing by ABI Prism 3130 (Applied Biosystems) as previously described [26] and evaluated with the Sequencing Analysis software. Each sequence reaction was carried out at least twice, starting from independent PCRs. In each case, the detected mutation was confirmed in the sequence as sense and antisense strands.
Statistical analyses
A two-tailed Fisher’s exact test was used to calculate the p values for the association among the variables. The level of significance was set at p < 0.05. The DFS and OS analyses were performed according to the Kaplan–Meier method, and survival curves were compared using the log-rank test. Data were analyzed using the IBM SPSS Statistics 20 package.
Results
Patients’ characteristics
Sixty-eight patients fulfilled the selection criteria and were included in this analysis. The median age at diagnosis was 69 years (range 30–74). The study population included 36 males and 32 females. The patients were at high risk for recurrence: 35 % were stage II and 65 % were stage III, respectively.
The mean number of delivered courses was 5 (range 4–6). Disease relapse accounted for all deaths (42 events). With a median follow-up of 40.5 months (range 4–163), DFS and OS were 18 (95 % CI 13.4–22.7) and 56 (95 % CI 44.8–67.1) months, respectively.
Expression of ERCC1 and TS, and mutation of p53
Immunohistochemistry analysis was performed in 67 cases (one case was not suitable for analysis due to the lack of staining at internal positive control). ERCC1 expression was categorized as score 0 in 14 out of 67 (21 %) cases, 1+ in 19 (28 %), 2+ in 20 (30 %) and 3+ in 14 cases (21 %). By using the H-score, the median value was 0.25 (range 0–3). Positive cases were considered those exhibiting an H-score higher than the median value (32 cases, 48 %). TS expression was categorized as score 0 in 17 out of 67 (25 %) cases, 1+ in 27 (40 %), 2+ in 16 (24 %) and 3 + in 8 (12 %) cases. By using the H-score, the median value was 0.15 (range 0–3). Positive cases were considered those exhibiting an H-score higher than the median value (30 cases, 45 %).
The analysis of p53 was feasible in 66 patients, since in two cases, amplification was not successful. Among these, an inactivating point mutation was observed in 20 (30 %) cases. The mutational spectrum was very broad, with the classical hotspot mutations observed in few cases (R175H in 3 cases, R273H in 2 cases and R248Q in 1 case). The polymorphism at codon 72 of p53 showed the sequence R72R in 52 (79 %) cases, R72P in 12 (18 %) cases and P72P in 2 (3 %) cases.
Molecular markers and clinicopathological correlations
Overall, no significant correlations were found between ERCC1 and TS protein expression, p53 mutations, p53 polymorphism in codon 72 and the clinicopathological parameters (gender, age and stage), except for the correlation of ERCC1 expression (1–3+) at IHC with stage III disease (p = 0.03). Accordingly, a multivariate analysis was not performed.
Molecular analysis and clinical outcome
The univariate analysis revealed no significant association between the molecular markers and relapse of disease. However, median OS was significantly longer in patients with ERCC1 negative tumors for IHC classified on the basis of the H-score (p = 0.04), while TS protein expression, p53 mutations and p53 polymorphism in codon 72 were not significantly associated with both DFS and OS. Table 1 summarizes the survival data according to molecular markers analyzed.
Discussion
Nowadays, the search for predictive biomarkers to define optimal treatment represents an intriguing challenge. Predictive markers could facilitate the selection of drugs best suited for each individual and allow physicians to avoid unnecessary toxicity and hospitalization to the patient, while preserving economical and human resources. Overall, this study evaluated the expression of key determinants for drug activity in gastric cancer specimens, particularly analyzing the relationship among ERCC1, TS and p53 alterations and clinical outcome in a cohort of patients treated with adjuvant ECF after curative D2 gastrectomy.
In gastric cancer, there are yet no validated molecular markers able to identify subgroups of patients who could benefit from these treatments. Outside clinical trials, in our institutions, it was common policy to offer adjuvant ECF to high-risk, curatively resected gastric cancer patients. To better investigate the role of adjuvant ECF in this setting, we focused on molecular alterations (ERCC1, TS and p53), for which a possible effect of platinum compounds and 5FU on clinical outcome has been suggested.
Excision repair cross-complementing gene 1 plays a pivotal role in the NER, which contributes in cisplatin resistance [27, 28]. In our study, the absence of expression of ERCC1 at IHC was found to correlate with better OS. Of note, no improvement in DFS was observed. This finding might be explained by the use of salvage platinum-based regimens in relapsed patients, giving potentially an additional advantage in ERCC1 negative tumors.
Our findings confirm the knowledge that an hyperactive NER (which occurs, for example, in the presence of ERCC1 overexpression) is able to repair the DNA breaks induced by the administration of platinum compounds, preventing cell death due to the DNA damage and leading, at last, to resistance to these compounds [29]. The association of ERCC1 and clinical outcome in patients treated with platinum-based regimens has already been described by other authors. Consistent with our results, Liu et al. [30] showed a longer OS in patients with low ERCC1 levels treated with adjuvant oxaliplatin-based chemotherapy. In contrast with these results, several studies demonstrate that ERCC1 overexpression correlates with better survival in curatively resected gastric cancer patients treated with adjuvant platinum-based regimens [31–33]. In addition, in patients treated with platinum-based regimens, low ERCC1 expression by IHC was associated with a higher response rate and survival [15–34]. Table 2 summarizes the studies on correlation of ERCC1 and outcome in curatively resected gastric cancer patients treated with platinum-based adjuvant chemotherapy. Discrepancy of our results with other studies could be attributed to different antibodies utilized within IHC protocols, the H-score and different cutoff used at IHC evaluation, as no standardized protocols and evaluation systems have been defined. Also ethnicity could play a major role when discussing our results not being consistent with some publications, as there is limited experience in Western population. Nonetheless, our investigation was conducted utilizing already published antibodies and protocols standardized for lung cancer, where the predictive role of ERCC1 is better depicted as compared to gastric cancer [24]. In addition, heterogeneity in chemotherapy regimens reported in the literature might also account for these differences in outcome. Finally, genetic polymorphisms have been shown to be closely associated with clinical outcome in patients treated with platinum/5FU-based chemotherapy, as multiple genes are involved in drug metabolism with complex interplay [35]. The enzyme TS has been associated with resistance to fluoropyrimidines, and p53 gene is thought to modulate the cytotoxic effects of 5FU, doxorubicin and cisplatin. We found no significant correlation between TS, p53 and clinical outcome. In gastric cancer, the role of TS in predicting chemosensitivity remains controversial. However, in advanced disease, high levels of TS have been correlated with resistance to fluoropyrimidines and poor outcome [36]. We observed that in adjuvant setting, TS expression did not correlate with the outcome in patients treated with 5FU chemotherapy [37, 38]; however, these data are in contrast with other reports [39]. In our cohort of gastric cancer patients treated with a 5FU-based adjuvant regimen, TS was not found to predict outcome.
Finally, the tumor suppressor p53 is speculated to modulate the cytotoxic effects of 5FU, doxorubicin and cisplatin [21, 40]. In advanced gastric cancer, there are conflicting results of p53 expression in predicting response to chemotherapy [41–44]: in patients treated with adjuvant 5FU-based chemotherapy, p53 overexpression was found as a negative independent predictive factor of survival [45]. On the contrary, our results revealed that the presence of p53 mutations turned out to be not associated with survival.
In conclusion, our data demonstrates that patients exhibiting low ERCC1 expression are more likely to benefit from platinum-based adjuvant chemotherapy, in a European population. If confirmed in a prospective study, this critical finding could lead to use alternative chemotherapy regimens in patients with ERCC1 overexpressed tumors, providing a more adequate treatment while avoiding unnecessary toxicities.
References
Songun I, Putter H, Kranenbarg EM et al (2010) Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 11:439–449
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN et al (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725–730
Macdonald J, Smalley S, Haller D, et al (2009) Chemoradiation of resected gastric cancer: a 10-year follow-up of the phase III trial INT0116 (SWOG 9008). J Clin Oncol 27(15 suppl), abstr 4515
Okines AF, Cunningham D (2010) Multimodality treatment for localized gastro-oesophageal cancer. Ann Oncol 21(Suppl 7):vii286–vii293
Hermans J, Bonenkamp JJ, Boon MC et al (1993) Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol 11:1441–1447
Earle CC, Maroun JA (1999) Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomized trials. Eur J Cancer 35:1059–1064
Mari E, Floriani I, Tinassi A, Buda A, Belfiglio M, Valentini M et al (2000) Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomized trials. Ann Oncol 11:837–843
Panzini I, Gianni L, Fattori PP et al (2002) Adjuvant chemotherapy in gastric cancer: a meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori 88:21–27
Paoletti X, Oba K, Burzykowski T et al (2010) Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group. JAMA 303:1729–1737
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Sasako M, Sakuramoto S, Katai H et al (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29:4387–4393
Bang YJ, Kim YW, Yang HK et al (2012) Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 379:315–321
Metzger R, Leichman CG, Danenberg KD et al (1998) ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol 16:309–316
Matsubara J, Nishina T, Yamada Y et al (2008) Impacts of excision repair cross-complementing gene 1 (ERCC1), dihydropyrimidine dehydrogenase, and epidermal growth factor receptor on the outcomes of patients with advanced gastric cancer. Br J Cancer 98:832–839
Yeh KH, Shun CT, Chen CL et al (1998) High expression of thymidylate synthase is associated with the drug resistance of gastric carcinoma to high dose 5-fluorouracil-based systemic chemotherapy. Cancer 82:1626–1631
Hua D, Huang ZH, Mao Y et al (2007) Thymidylate synthase and thymidine phosphorylase gene expression as predictive parameters for the efficacy of 5-fluorouracil-based adjuvant chemotherapy for gastric cancer. World J Gastroenterol 13:5030–5034
Choi J, Lim H, Nam DK et al (2001) Expression of thymidylate synthase in gastric cancer patients treated with 5-fluorouracil and doxorubicin-based adjuvant chemotherapy after curative resection. Br J Cancer 84:186–192
Lenz HJ, Leichman CG, Danenberg KD et al (1996) Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumor response and overall survival. J Clin Oncol 14:176–182
Chin KV, Ueda K, Pastan I et al (1992) Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science 255:459–462
Lowe SW, Ruley HE, Jacks T et al (1993) p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74:957–967
Findlay M, Cunningham D, Norman A et al (1994) A phase II study in advanced gastro-esophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF). Ann Oncol 5:609–616
van Krieken JH, Jung A, Kirchner T et al (2008) KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: proposal for an European quality assurance program. Virchows Arch 453:417–431
Olaussen KA, Dunant A, Fouret P et al (2006) DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 355:983–991
Zucali AP, Giovannetti E, Destro A et al (2011) Thymidylate synthase and excision repair cross-complementing group-1 as predictors of responsiveness in mesothelioma patients treated with pemetrexed/carboplatin. Clin Cancer Res 17:2581–2590
Perrone F, Oggionni M, Birindelli S et al (2003) TP53, p14ARF, p16INK4a and H-ras gene molecular analysis in intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Int J Cancer 105:196–203
Rosell R, Taron M, Ariza A et al (2004) Molecular predictors of response to chemotherapy in lung cancer. Semin Oncol 31(1 Suppl 1):20–27
Dabholkar M, Bostick-Bruton F, Weber C et al (1992) ERCC1 and ERCC2 expression in malignant tissues from ovarian cancer patients. J Natl Cancer Inst 84:1512–1517
Reed E (2005) ERCC1 and clinical resistance to platinum-based therapy. Clin Cancer Res 11:6100–6102
Liu Y, Ling Y, Liu B et al (2010) Use of ERCC1 expression in resected specimen to predict survival in both patients with gastric cancer treated with surgery followed by oxaliplatin-based adjuvant chemotherapy and in those receiving surgery alone. Gastrointestinal Cancers Symposium abstr 29
Kim KH, Kwon HC, Oh SY et al (2011) Clinicopathologic significance of ERCC1, thymidylate synthase and glutathione S-transferase P1 expression for advanced gastric cancer patients receiving adjuvant 5-FU and cisplatin chemotherapy. Biomarkers 16:74–82
Baek SK, Kim SY, Lee JJ et al (2006) Increased ERCC expression correlates with improved outcome of patients treated with cisplatin as an adjuvant therapy for curatively resected gastric cancer. Cancer Res Treat 38:19–24
Bamias A, Karina M, Papakostas P et al (2010) A randomized phase III study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer Chemother Pharmacol 65:1009–1021
Kwon HC, Roh MS, Oh SY et al (2007) Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol 18:504–509
Wang Z, Chen JQ, Liu JL et al (2012) Polymorphisms in ERCC1, GSTs, TS and MTHFR predict clinical outcomes of gastric cancer patients treated with platinum/5-Fu-based chemotherapy: a systematic review. BMC Gastroenterol 12:137
Yeh KH, Shun CT, Chen CL et al (1998) High expression of thymidylate synthase is associated with the drug resistance of gastric carcinoma to high dose 5-fluorouracil-based systemic chemotherapy. Cancer 82:1626–1631
Kim JS, Kim MA, Kim TM et al (2009) Biomarker analysis in stage III-IV (M0) gastric cancer patients who received curative surgery followed by adjuvant 5-fluorouracil and cisplatin chemotherapy: epidermal growth factor receptor (EGFR) associated with favourable survival. Br J Cancer 100:732–738
Choi J, Lim H, Nam DK et al (2001) Expression of thymidylate synthase in gastric cancer patients treated with 5-fluorouracil and doxorubicin-based adjuvant chemotherapy after curative resection. Br J Cancer 84:186–192
Hua D, Huang ZH, Mao Y et al (2007) Thymidylate synthase and thymidine phosphorylase gene expression as predictive parameters for the efficacy of 5-fluorouracil-based adjuvant chemotherapy for gastric cancer. World J Gastroenterol 13:5030–5034
Chin KV, Ueda K, Pastan I et al (1992) Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science 255:459–462
Boku N, Chin K, Hosokawa K et al (1998) Biological markers as a predictor for response and prognosis of unresectable gastric cancer patients treated with 5-fluorouracil and cis-platinum. Clin Cancer Res 4:1469–1474
Yeh KH, Shun CT, Chen CL et al (1999) Overexpression of p53 is not associated with drug resistance of gastric cancers to 5-fluorouracil-based systemic chemotherapy. Hepatogastroenterology 46:610–615
Giatromanolaki A, Stathopoulos GP, Koukourakis MI et al (2001) Angiogenesis and apoptosis-related protein (p53, bcl-2, and bax) expression versus response of gastric adenocarcinomas to paclitaxel and carboplatin chemotherapy. Am J Clin Oncol 24:222–226
Kikuyama S, Inada T, Shimizu K et al (2001) p53, bcl-2 and thymidine phosphorylase as predictive markers of chemotherapy in patients with advanced and recurrent gastric cancer. Anticancer Res 21:2149–2153
Díez M, Medrano MJ, Gutierrez A et al (2000) P53 protein expression in gastric adenocarcinoma. Negative predictor of survival after postoperative adjuvant chemotherapy. Anticancer Res 20:3929–3933
Acknowledgments
The work was supported by Fondo di Ricerca Ente Ospedaliero Cantonale.
Conflict of interest
All the authors have no disclosures to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Piercarlo Saletti and Milo Frattini are senior co-authors.
Rights and permissions
About this article
Cite this article
De Dosso, S., Zanellato, E., Nucifora, M. et al. ERCC1 predicts outcome in patients with gastric cancer treated with adjuvant cisplatin-based chemotherapy. Cancer Chemother Pharmacol 72, 159–165 (2013). https://doi.org/10.1007/s00280-013-2181-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2181-2