Abstract
Purpose
There is as yet no optimal treatment regimen for patients with epidermal growth factor receptor (EGFR) gene wild-type non-small-cell lung cancer (NSCLC) that has progressed despite cytotoxic chemotherapy. This trial was performed to evaluate the efficacy and toxicity of erlotinib, a tyrosine kinase inhibitor of EGFR, in Japanese patients with EGFR wild-type tumors.
Methods
Patients with stage III/IV or postoperative recurrence of NSCLC whose tumors have wild-type EGFR were eligible. Erlotinib (150 mg/day) was administered until disease progression or unacceptable toxicity occurred. The primary end point was disease control rate (DCR).
Results
Thirty-one patients (23 men and 8 women; median age, 71 years; range, 31–89) were enrolled between January 2008 and June 2011. Twenty-one had adenocarcinoma, nine had squamous cell carcinoma, and one had large cell carcinoma. Ten, nine, eight, and four patients showed performance status 0, 1, 2, and 3, respectively. Erlotinib was administered following the median 3.1 regimens of cytotoxic chemotherapies. One patient achieved complete response, four showed partial response, and eight had stable disease. Thus, response rate was 17.2%, and DCR was 44.8%. Skin rash was the most common side effect (80.6%). Two patients developed interstitial lung disease. Nevertheless, all of these events were reversible, and there were no treatment-related deaths. The median progression-free survival and survival times were 2.1 and 7.7 months, respectively.
Conclusion
Erlotinib might be an alternative option for patients resistant to cytotoxic chemotherapy even in those with EGFR wild-type NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related death in Japan and throughout the Western world [1, 2]. Platinum-based doublet combinations are standard regimens for first-line treatment in advanced-staged non-small-cell lung cancer (NSCLC) and have provided only modest survival advantages [3, 4]. Tyrosine kinase inhibitors of the epidermal growth factor receptor (EGFR-TKIs) are promising therapeutic options for patients with NSCLC [5, 6], especially in Asia [7–12]. Erlotinib and gefitinib are selective EGFR-TKIs, and numerous clinical studies demonstrated favorable efficacy and toxicity profiles compared with cytotoxic chemotherapy [7, 8]. The efficacy of EGFR-TKIs is associated with EGFR-sensitive mutation status in NSCLC [5–9]. A high response rate (RR) to EGFR-TKIs is observed in patients with EGFR-sensitive mutations, but the RR is 1.0–13.9% in wild-type EGFR [8, 13–15].
In the Iressa Survival Evaluation in Lung Cancer (ISEL) study, however, gefitinib failed to prolong survival in unselected patients with advanced NSCLC after failure of at least one prior chemotherapy regimen [16]. However, in the same clinical setting study (BR.21) [17], erlotinib showed a survival advantage of 6.67 months for erlotinib versus 4.70 months for the placebo. Thus, erlotinib is the only EGFR-TKI shown to provide a survival benefit for advanced unselected NSCLC patients. In addition, several clinical studies indicated that erlotinib could confer benefits in certain patients with NSCLC after gefitinib failure [18, 19]. Thus, erlotinib may have a higher biological activity and distinct clinical outcomes from gefitinib [20, 21]. Based on these findings, it is speculated that when treatment with cytotoxic chemotherapies fails in patients with wild-type EGFR, erlotinib may be a suitable option for salvage therapy. There is as yet no optimal treatment regimen for patients with EGFR wild-type NSCLC that has progressed despite several rounds of cytotoxic chemotherapy.
Therefore, we performed this prospective study to investigate the efficacy and tolerability of erlotinib monotherapy in Japanese patients with wild-type EGFR as a potential therapeutic option in heavily pretreated NSCLC patients with progressive disease after treatment with cytotoxic agents.
Patients and methods
Patient eligibility
Patients eligible for this study were required to have histologically or cytologically proven stage III/IV or postoperative recurrent NSCLC without EGFR-sensitive mutations (exons 18, 19, and 21). The other inclusion criteria were (1) age ≥20 years old; (2) Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–3; (3) measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 [22]; (4) no prior history of EGFR-TKI therapy; and (5) adequate hepatic and renal function. Patients were excluded from this study for any of the following reasons: (1) receiving systemic anticancer therapy within 4 weeks; (2) past history of hypersensitivity to drugs; (3) severe complications; (4) active infection; (5) interstitial lung disease (ILD) detectable on chest radiography; (6) pleural, pericardial, or peritoneal effusion requiring drainage; (7) active brain metastasis; or (8) pregnancy. This study was approved by the institutional review boards of the participating institutes and was conducted according to the principles of the Declaration of Helsinki. All enrolled patients gave their written informed consent.
Pretreatment evaluation
Before enrollment in this study, all patients underwent clinical and physical examination: PS, medical history, routine laboratory tests, electrocardiography, chest radiography, computed tomography (CT) scan of the chest and abdomen, and magnetic resonance imaging (MRI) scan of the whole brain. Positron emission tomography/CT and isotope bone scan were performed if medically indicated. Histological or cytological specimens containing tumor cells were examined for EGFR mutations by the peptide nucleic acid-locked nucleic acid polymerase chain reaction (PNA-LNA PCR) clamp assay. This assay can detect mutated EGFR sequences with high specificity and sensitivity and is commercially available in Japan.
Treatment protocol
Erlotinib was taken orally at a dose of 150 mg daily. Erlotinib therapy was continued until disease progression (PD) or withdrawal of consent. Erlotinib was interrupted or a dose reduction considered in patients who developed grade 3 non-hematological toxicities or fever of ≥38.0°C. In addition, erlotinib was discontinued under any of the following conditions: (1) development of grade 1 ILD or any grade 4 toxicity and (2) interruption for over 2 weeks as a result of over grade 3 toxicity. During the trial, no other systemic anticancer treatment was permitted.
Toxicity and response evaluation
All toxicities were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 [23]. Chest radiography or CT scan was performed every 2–4 weeks to assess the response. Complete and partial responses were confirmed by two observations not less than 4 weeks apart. Determination of stable disease (SD) required disease stabilization for at least 6 weeks in the present study.
Statistical considerations
The primary end point was the disease control rate (DCR). The expected DCR was 60%, and threshold DCR was 33%. We estimated that a total of 29 patients would be needed for the study to have a power of 90% to confirm the hypothesis with a two-sided significance level of 5%. Secondary end points were RR, toxicities, progression-free survival (PFS), and overall survival (OS). OS was defined as the time from enrollment in this study until death from any cause. PFS was defined as the time from enrollment in this study to the first observation of PD or death from any cause. PFS and OS were analyzed by the Kaplan–Meier method and were compared using the log-rank test. The χ2 test was used for comparisons between two groups.
Results
Patient characteristics
A total of 31 patients were enrolled between January 2008 and June 2011 from six institutes in Nagano prefecture, Japan. The clinical characteristics of the patients are summarized in Table 1. The median age was 71 years, with a range of 31–89 years. Most of the patients were men (74.2%) and were smokers (77.4%). Histological types included 21 cases of adenocarcinoma (67.7%), nine of squamous cell carcinoma (29.0%), and one of large cell carcinoma (3.2%). Ten, nine, eight, and four patients showed PS 0, 1, 2, and 3, respectively. Two patients were treated with erlotinib as the first-line chemotherapy because of advanced age (76 and 84 years old). Eleven patients were treated with erlotinib as second-line therapy, and nine cases were treated as third-line therapy. Nine patients (29.0%) in the present study were treated with erlotinib as fourth-line or later therapy.
Toxicity and treatment delivery
The adverse event profile is summarized in Table 2. The most common adverse events associated with erlotinib treatment were skin rash (80.6%) and diarrhea (38.7%). Two patients (6.5%) developed ILD, but they recovered with steroid treatment. Hematological toxicity was not observed in this study. There were no treatment-related deaths in the whole study population. Median treatment duration was 70 days with a range of 10–463 days. Two patients discontinued erlotinib treatment before response evaluation because of the development of ILD and patient refusal, respectively. Dose reduction of erlotinib was performed in five patients (16.1%) because of toxicities (eruption or diarrhea).
Response and survival
The response to erlotinib was evaluated in all except two patients because of discontinuation of therapy before evaluation. The results are shown in Table 3. One patient achieved a complete response (CR), four showed a partial response (PR), and eight showed SD; thus, the RR was 17.2% (95% confidence interval (CI): 7.6–35.4%) and DCR was 44.8% (95% CI: 28.4–62.5%). We also analyzed the tumor response according to patients’ characteristics and adverse effects. Patients with a skin rash of grade 2–3 showed a significantly higher DCR (57.1%) than those with grade 0 or 1 rash (12.5%, P = 0.02). There were no significant differences in DCR in adenocarcinoma and squamous cell carcinoma groups.
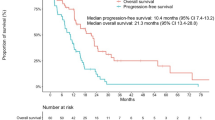
Survival was analyzed in all patients, and the survival curves are shown in Fig. 1. The median PFS and median survival time (MST) were 2.1 months (95% CI: 0.9–2.8 months) and 7.7 months (3.8–20.4 months), respectively. One-year survival rate was 44.2% (95% CI: 26.2–63.9%). The PFS and OS in patients with CR + PR + SD were significantly longer than in those with PD (Fig. 2). In addition, patients with PS 3 had significantly shorter PFS (0.4 months) and OS (0.9 months) than in those with PS 0–2 (PFS: 2.2 months, P = 0.0002 and OS: 10.3 months, P = 0.0002). No significance was observed in subgroups: gender, smoking history, and adverse effects. Patients with grade 2–3 rash showed longer PFS and OS than the group with grade 0 or 1 rash, but the difference was not significant (PFS, P = 0.15; OS, P = 0.06). Furthermore, survival tended to be longer in the adenocarcinoma group than the squamous cell carcinoma group, but the difference was not significant (P = 0.11).
Kaplan–Meier plot of progression-free survival (a) and overall survival (b) after enrollment in the study according to the response. Positive responders (CR + PR + SD) had longer median PFS (4.0 months, 95% CI: 2.6–10.9) than non-responders (0.9 months, 95% CI: 0.4–1.7, P = 0.0001). Positive responders also had longer median OS (20.4 months, 95% CI: 6.7–not reached) than non-responders (4.8 months, 95% CI: 1.7–14.6, P = 0.009)
Discussion
We prospectively evaluated the efficacy and toxicities of erlotinib in patients with EGFR wild-type NSCLC. In the present study, we found an objective RR of 17.2%, a median PFS time of 2.1 months, and MST of 7.7 months, along with manageable and non-fatal toxicities. As most patients enrolled in the present study had already received cytotoxic chemotherapy, the RR of 17.2% and DCR of 44.9% were encouraging results. Thus, we suggest that EGFR-TKI using erlotinib may be an alternative option for patients resistant to cytotoxic chemotherapy, even in those with EGFR wild-type NSCLC.
Wu et al. [14] retrospectively summarized the effectiveness of erlotinib in patients with EGFR wild-type NSCLC and described the RR of 13.9%. In addition, Schneider et al. [15] also analyzed the patients from German Center in TRUST study [24] and reported a 3% response to erlotinib in EGFR wild-type cases. Yoshioka et al. [25] conducted a phase II study prospectively and reported RR of 3% and DCR of 60% to erlotinib in Japanese patients with EGFR wild-type NSCLC. Compared with these results, the response rate to erlotinib in the present study was somewhat higher, although DCR and PFS were almost identical to these previous reports. As the number of patients was small in all of these studies, including the present study, it is difficult to interpret the differences. Tumor tissues in NSCLC can include histologically heterogeneous components and detection of positive or resistant EGFR mutant tumor cells may vary among different tumor sites [26, 27]. As EGFR mutations were determined at initial diagnosis and not at initiation of erlotinib treatment, the biological features in the various sites of tumors may have changed somewhat during cytotoxic chemotherapies. Reevaluation of EGFR mutation may help to determine the variability in the tumors.
The DCR of 44.8% obtained here suggests that treatment with erlotinib would have a significant effect on the clinical course of patients with EGFR wild-type NSCLC. We cannot exclude the possibility that the efficacy of erlotinib observed in the present study may reflect the natural history of the disease rather than the efficacy of the drug. However, the duration of median PFS (4.0 months) in patients obtained over SD was substantial. The appropriate treatment in EGFR wild-type NSCLC resistant to several cytotoxic chemotherapies has yet to be determined. As we have encountered many patients with no further treatment options who have progressed despite receiving several cytotoxic chemotherapies, we emphasize that erlotinib may be a useful optional treatment for patients with EGFR wild-type tumors.
In a retrospective analysis comparing the effectiveness of erlotinib and gefitinib in patients with EGFR wild-type NSCLC, there are no differences in response rate or survival rate between the two regimens [14]. As the present study focused on the efficacy of erlotinib, the superiority or at least non-inferiority of erlotinib to gefitinib in cases resistant to multiple cytotoxic chemotherapy regimens was not determined.
It is difficult to clarify the molecular mechanisms underlying the effectiveness of erlotinib in this patient population. Recently, Chang et al. [28] analyzed the expression of amphiregulin, a novel molecular biomarker, in patients with EGFR wild-type NSCLC who were treated with EGFR-TKIs. They reported that, although the relationship with DCR was not statistically significant, positive amphiregulin status using immunohistochemical staining was associated with prolonged PFS and OS. Thus, amphiregulin could be a potential marker for the selection of EGFR-TKI treatment in patients with EGFR wild-type NSCLC. Thus, further studies are warranted to evaluate the molecular mechanism and clarify how to select patients for erlotinib treatment among those with EGFR wild-type NSCLC.
In conclusion, erlotinib is a potentially useful therapeutic option for advanced NSCLC patients with EGFR wild-type tumors showing resistance to cytotoxic chemotherapy. Although the molecular mechanisms underlying the observations of the present study remain unclear, the results presented here suggest that erlotinib has some clinical efficacy even in patients with EGFR wild-type NSCLC.
References
Matsuda T, Marugame T, Kamo K, Katanoda K, Ajiki W, Sobue T, The Japan Cancer Surveillance Research Group (2011) Cancer incidence and incidence rates in Japan in 2005: based on data from 12 population-based cancer registries in the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol 41:139–147
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, Nordquist L, Pfister DG, Piantadosi S, Schiller JH, Smith R, Smith TJ, Strawn JR, Trent D, Giaccone G, American Society of Clinical Oncology (2009) American society of clinical oncology clinical practice guideline update on chemotherapy for stage IV non-small cell lung cancer. J Clin Oncol 27:6251–6266
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Eastern Cooperative Oncology Group (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L, National Cancer Institute of Canada Clinical Trials Group (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Johnson JR, Cohen M, Sridhara R, Chen YF, Williams GM, Duan J, Gobburu J, Booth B, Benson K, Leighton J, Hsieh LS, Chidambaram N, Zimmerman P, Pazdur R (2005) Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res 11:6414–6421
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T, North-East Japan Study Group (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Inoue A, Suzuki T, Fukuhara T, Maemondo M, Kimura Y, Morikawa N, Watanabe H, Saijo Y, Nukiwa T (2006) Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol 24:3340–3346
Mitsudomi T, Yatabe Y (2007) Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 98:1817–1824
Morita S, Okamoto I, Kobayashi K, Yamazaki K, Asahina H, Inoue A, Hagiwara K, Sunaga N, Yanagitani N, Hida T, Yoshida K, Hirashima T, Yasumoto K, Sugio K, Mitsudomi T, Fukuoka M, Nukiwa T (2009) Combined survival analysis of prospective clinical trials of gefitinib for non-small cell lung cancer with EGFR mutations. Clin Cancer Res 15:4493–4498
Asami K, Koizumi T, Hirai K, Ameshima S, Tsukadaira A, Morozumi N, Morikawa A, Atagi S, Kawahara M (2011) Gefitinib as first-line treatment in elderly epidermal growth factor receptor-mutated patients with advanced lung adenocarcinoma: results of a Nagano Lung Cancer Research Group Study. Clin Lung Cancer 12:387–392
Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu DT, Saijo N, Duffield EL, Rukazenkov Y, Speake G, Jiang H, Armour AA, To KF, Yang JC, Mok TS (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29:2866–2874
Wu JY, Wu SG, Yang CH, Chang YL, Chang YC, Hsu YC, Shih JY, Yang PC (2011) Comparison of gefitinib and erlotinib in advanced NSCLC and the effect of EGFR mutations. Lung Cancer 72:205–212
Schneider CP, Heigener D, Schott-von-Römer K, Gütz S, Laack E, Digel W, Guschall WR, Franke A, Bodenstein H, Schmidtgen C, Reck M (2008) Epidermal growth factor receptor-related tumor markers and clinical outcomes with erlotinib in non-small cell lung cancer: an analysis of patients from german centers in the TRUST study. J Thorac Oncol 3:1446–1453
Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V, Carroll K (2005) Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366:1527–1537
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L, National Cancer Institute of Canada Clinical Trials Group (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Cho BC, Im CK, Park MS, Kim SK, Chang J, Park JP, Choi HJ, Kim YJ, Shin SJ, Sohn JH, Kim H, Kim JH (2007) Phase II study of erlotinib in advanced non-small-cell lung cancer after failure of gefitinib. J Clin Oncol 25:2528–2533
Lee DH, Kim SW, Suh C, Yoon DH, Yi EJ, Lee JS (2008) Phase II study of erlotinib as a salvage treatment for non-small-cell lung cancer patients after failure of gefitinib treatment. Ann Oncol 19:2039–2042
Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, DiOrio C, Doty J, Morin MJ, Moyer MP, Neveu M, Pollack VA, Pustilnik LR, Reynolds MM, Sloan D, Theleman A, Miller P (1997) Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res 57:4838–4848
Paz-Ares L, Soulières D, Melezínek I, Moecks J, Keil L, Mok T, Rosell R, Klughammer B (2010) Clinical outcomes in non-small-cell lung cancer patients with EGFR mutations: pooled analysis. J Cell Mol Med 14:51–69
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2010) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Common terminology criteria for adverse events (CTCAE) Version 3.0. National Cancer Institute Web site. 22 May 2003. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 1 March 2011
Reck M, van Zandwijk N, Gridelli C, Baliko Z, Rischin D, Allan S, Krzakowski M, Heigener D (2010) Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol 5:1616–1622
Yoshioka H, Hotta K, Kiura K, Takigawa N, Hayashi H, Harita S, Kuyama S, Segawa Y, Kamei H, Umemura S, Bessho A, Tabata M, Tanimoto M, Okayama Lung Cancer Study Group (2010) A phase II trial of erlotinib monotherapy in pretreated patients with advanced non-small cell lung cancer who do not possess active EGFR mutations: Okayama Lung Cancer Study Group trial 0705. J Thorac Oncol 5:99–104
Bozzetti C, Tiseo M, Lagrasta C, Nizzoli R, Guazzi A, Leonardi F, Gasparro D, Spiritelli E, Rusca M, Carbognani P, Majori M, Franciosi V, Rindi G, Ardizzoni A (2008) Comparison between epidermal growth factor receptor (EGFR) gene expression in primary non-small cell lung cancer (NSCLC) and in fine-needle aspirates from distant metastatic sites. J Thorac Oncol 3:18–22
Ushiki A, Koizumi T, Kobayashi N, Kanda S, Yasuo M, Yamamoto H, Kubo K, Aoyagi D, Nakayama J (2009) Genetic heterogeneity of EGFR mutation in pleomorphic carcinoma of the lung: response to gefitinib and clinical outcome. Jpn J Clin Oncol 39:267–270
Chang MH, Ahn HK, Lee J, Jung CK, Choi YL, Park YH, Ahn JS, Park K, Ahn MJ (2011) Clinical impact of amphiregulin expression in patients with epidermal growth factor receptor (EGFR) wild-type nonsmall cell lung cancer treated with EGFR-tyrosine kinase inhibitors. Cancer 117:143–151
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, T., Koizumi, T., Agatsuma, T. et al. A phase II trial of erlotinib in patients with EGFR wild-type advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 69, 1241–1246 (2012). https://doi.org/10.1007/s00280-012-1831-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1831-0