Abstract
Purpose
The glucose analog and glycolytic inhibitor 2-deoxy-d-glucose (2-DG), which is currently under clinical evaluation for targeting cancer cells, not only blocks glycolysis thereby reducing cellular ATP, but also interferes with N-linked glycosylation, which leads to endoplasmic reticulum (ER) stress and an unfolded protein response (UPR). Both bioenergetic challenge and ER stress have been shown to activate autophagy, a bulk cellular degradation process that plays either a pro- or anti-death role. Here, we investigate which pathway 2-DG interferes with that activates autophagy and the role of this process in modulating 2-DG-induced toxicity.
Methods
Pancreatic cancer cell line 1420, melanoma cell line MDA-MB-435 and breast cancer cell line SKBR3 were used to investigate the relationship between induction by 2-DG treatment of ER stress/UPR, ATP reduction and activation of autophagy. ER stress/UPR (Grp78 and CHOP) and autophagy (LC3B II) markers were assayed by immunoblotting, while ATP levels were measured using the CellTiter-Glo Luminescent Cell Viability Assay. Autophagy was also measured by immunofluorescence utilizing LC3B antibody. Cell death was detected with a Vi-Cell cell viability analyzer using trypan blue exclusion.
Results
In the three different cancer cell lines described earlier, we find that 2-DG upregulates autophagy, increases ER stress and lowers ATP levels. Addition of exogenous mannose reverses 2-DG-induced autophagy and ER stress but does not recover the lowered levels of ATP. Moreover, under anaerobic conditions where 2-DG severely depletes ATP, autophagy is diminished rather than activated, which correlates with lowered levels of the ER stress marker Grp78. Additionally, when autophagy is blocked by siRNA, cell sensitivity to 2-DG is increased corresponding with upregulation of ER stress-mediated apoptosis. Similar increased toxicity is observed with 3-methyladenine, a known autophagy inhibitor. In contrast, rapamycin which enhances autophagy reduces 2-DG-induced toxicity.
Conclusions
Overall, these results indicate that the major mechanism by which 2-DG stimulates autophagy is through ER stress/UPR and not by lowering ATP levels. Furthermore, autophagy plays a protective role against 2-DG-elicited cell death apparently by relieving ER stress. These data suggest that combining autophagy inhibitors with 2-DG may be useful clinically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
2-Deoxy-d-glucose (2-DG) is a glucose analog that interferes with glycolysis by blocking the first two reactions in this pathway carried out by hexokinase (HK) and phosphoglucose isomerase (PGI) [1, 2]. When glycolysis is inhibited in the presence of O2, cells can still produce ATP through their mitochondria using alternative energy sources, i.e., amino and fatty acids. Thus, 2-DG only induces sublethal levels of ATP reduction and does not kill most tumor cell types under normal O2 tension. However, under hypoxia and anaerobic conditions (cells treated with the mitochondrial ATP synthase inhibitor oligomycin or ρ0 cells that are devoid of mitochondrial DNA), oxidative phosphorylation in mitochondria is inhibited or reduced and hence cells rely mainly on glycolysis for energy production. Under these conditions, in contrast to normoxia, 2-DG severely depletes ATP, eventually leading to massive cell death [3–5]. This selective toxicity of 2-DG toward hypoxic cells has led to a Phase I clinical trial using 2-DG in combination with chemotherapy in order to target slow-growing hypoxic tumor cells and fast-proliferating normoxic tumor cells, respectively [6].
Recently, however, our laboratory has shown that 2-DG can also kill a subset of cancer cell lines under normal O2 conditions by inducing endoplasmic reticulum (ER) stress [7]. Due to its structural similarity to mannose, 2-DG interferes with oligosaccharide synthesis leading to abnormal N-linked glycosylation [8, 9] resulting in ER stress. In support of this hypothesis, mannose, the main sugar comprising the oligosaccharide chain in N-linked glycosylation, reversed 2-DG-induced ER stress and cell death whereas glucose at a similar concentration did not. Moreover, by the nature of its structure, 2-fluoro-deoxy-d-glucose (2-FDG) inhibits glycolysis more potently than 2-DG. However, it mimics mannose less closely than 2-DG does and thereby has been shown to be less potent than 2-DG in inducing ER stress and cytotoxicity [10]. Cells initially cope with ER stress through the unfolded protein response (UPR), a mechanism which attempts to relieve the ER burden by inhibiting global protein translation and thereby reducing the amount of proteins entering the ER, increasing degradation of misfolded/unfolded proteins and enhancing the ER folding capacity (upregulating protein chaperones). These UPR responses are mediated, respectively, through activation of the ER-resident transmembrane proteins, PKR-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1) and activating transcription factor 6 (ATF6). When the UPR is persistent, apoptosis is activated via C/EBP homologous protein—(CHOP), c-Jun N-terminal kinase—(JNK) and/or caspase 4—(caspase 12 in murine cells)-mediated mechanisms [11, 12].
In addition to the UPR response, it has recently been appreciated that ER stress also induces the well-described and studied process of autophagy [13–19] which is considered to be a cell survival mechanism previously shown to be activated in response to nutrient deprivation. During this process, an isolated double membrane structure is formed and expands to encompass cytoplasmic contents and/or cellular organelles, and finally comes to a closure to become an autophagosome. This structure then fuses with a lysosome to form the so-called autolysosome, where its contents are degraded and recycled for use by cells under stress [20, 21]. Although autophagy has been shown to be activated by environmental conditions and/or drugs that lower ATP [22–28] or induce ER stress, it remains unclear which of these processes is responsible for autophagy activation when cells are treated with 2-DG.
Here, we find that 2-DG-induced autophagy is abolished by addition of exogenous mannose that reverses 2-DG-induced ER stress without affecting lowered ATP levels. Moreover, the classical ER stress inducer tunicamycin is found to stimulate autophagy in a similar manner as that of 2-DG. In contrast, oxamate, which inhibits glycolysis and reduces ATP without causing ER stress, does not activate autophagy in the cell types examined here. Interestingly, a correlation between reduced autophagic activity and diminished ER stress/UPR marker is observed when cells are treated by 2-DG under anaerobic conditions (oligomycin treated). Additionally, we also demonstrate that enhancing autophagic capacity protects cells from 2-DG-related cytotoxicity, while inhibition of this process increases 2-DG-induced ER stress and apoptosis. Together, our results suggest that 2-DG-induced autophagy is activated mainly via ER stress/UPR, and not through ATP depletion, and that it plays a protective role against 2-DG-elicited cell death by relieving ER stress. The intention of this study is to further clarify the anti-tumor mechanisms of this sugar analog in order to improve its clinical performance, potentially through combination with autophagy inhibitors.
Materials and methods
Cell types
Human tumor cell lines 1420 (pancreatic) and MDA-MB-435 (melanoma) [29] were purchased from American Type Culture Collection (ATCC), and maintained in DMEM with 1 g/l of glucose (Mediatech). Human breast cancer cell line SKBR3 was a gift from Dr. Joseph Rosenblatt (University of Miami; Miami, FL) and maintained in RPMI with 2 g/l of glucose (Invitrogen). All culture media were supplemented with 10% fetal bovine serum (FBS) (Invitrogen), penicillin/streptomycin (Invitrogen) and 0.1% Plasmocin (only for cell passage) (InvivoGen) unless otherwise specified. Cells were grown under 5% CO2 at 37°C.
Drugs and antibodies
2-DG, mannose, tunicamycin, oxamate, oligomycin, 3-MA and rapamycin were purchased from Sigma–Aldrich. EST (E64d) was obtained from Calbiochem. The following rabbit primary antibodies were from Cell Signaling: Grp78, LC3B, total ACC, phosphorylated ACC (serine 79), Atg7 and cleaved caspase 3. Mouse anti-CHOP and anti-β-actin antibodies were from Cell Signaling and Sigma–Aldrich, respectively. Horseradish peroxidase (HRP)-conjugated anti-rabbit and anti-mouse IgG were purchased from Promega, and Alexa Fluor 488 goat anti-rabbit IgG was obtained from Invitrogen.
ATP quantification
Intracellular ATP levels were measured with the CellTiter-Glo Luminescent Cell Viability Assay (Promega) according to the manufacturer’s directions. Briefly, cells were seeded onto 96-well plates and cultured for 18–22 h to reach ~70% confluence. After 5 h of drug exposure, cells were lysed in the same plate with the reagent included in the assay kit for 10 min. Then the mixtures were transferred onto opaque-walled 96-well plates, and luminescence produced from ATP-mediated chemical reaction was read by the luminescence module of the FLUOstar OPTIMA microplate reader (BMG LABTECH). Readouts from control samples were set as 100% and those from all the other samples were presented as percentages of controls. Results were the averages of triplicate samples +SD from one representative experiment out of at least three independent analyses. Short-time (5 h) treatment was employed to minimize the toxicity and cell number loss caused by drug exposure.
Immunoblotting analysis
Cells were seeded onto six-well plates and cultured for 18–22 h to reach 40–70% confluence. Following drug exposure for the indicated times, cells were harvested and lysed with the lysis buffer (100 mM Tris–HCl at pH 7.4, 1% SDS, phosphatase inhibitor cocktail 2 and protease inhibitor cocktail from Sigma–Aldrich). Protein concentrations of each sample were determined using a Micro BCA Protein Assay Kit (Thermo Scientific) according to the manufacturer’s directions, and equal amounts of proteins were loaded onto 4–15% Tris–HCl gradient gels (except 12% gels for siRNA experiments) (Bio-Rad). After SDS–PAGE, proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore), blocked with 5% milk and probed with corresponding primary antibodies overnight (except 1 h for β-actin). The membrane was washed and probed with secondary antibodies for 1 h. Membrane was then incubated with SuperSignal West Pico or Femto Chemiluminescent Substrate (Thermo Scientific) and signals were visualized on Blue Lite Autorad Films (ISCBioExpress). All primary antibodies were used at 1:1000 dilution except for Atg7 (1:300) and β-actin (1:10000), and the secondary antibodies were used at 1:10000. Representative blots from at least three independent experiments were shown unless otherwise indicated.
Fluorescent microscopy
Cells were seeded onto eight-well Lab-Tek II Cc chamber slides (Thomas Scientific) and cultured for 18–22 h to reach ~60% confluence. After 16 h of drug exposure, cells were fixed with 10% neutral-buffered formalin solution containing 4% formaldehyde (Sigma–Aldrich) and permeabilized with methanol at −20°C. Following blocking with 5% normal goat serum (Dako), cells were incubated with anti-LC3B antibody (1:400) overnight. Then, cells were washed and incubated with Alexa Fluor 488 goat anti-rabbit IgG (1:500) for 1 h, mounted with ProLong Gold antifade reagent with DAPI (Invitrogen) and visualized with the Laborlux fluorescent microscope (Leitz) equipped with a DFC 340 FX digital camera (Leica). Individual cells were shown from one representative experiment out of at least three independent analyses unless otherwise indicated.
Cytotoxicity assay
Cells were seeded onto 24-well plates and cultured for 18–22 h. After drug exposure for 48 or 72 h, attached cells and their respective culture media were collected and centrifuged at 400g for 5 min. The pellets were then resuspended in Hanks Balanced Salt Solution (HBSS) (Mediatech) and analyzed with Vi-Cell cell viability analyzer (Beckman Coulter) based on trypan blue exclusion. Results were shown as the percentages of dead cells out of total cells counted. Data were the averages of triplicate samples +SD from one representative experiment out of at least three independent analyses unless otherwise indicated.
siRNA transfection
Cells were seeded into 25-cm2 flasks and cultured for 24 h to reach ~60% confluence using antibiotics-free media. Then, cells were transfected with anti-Luc siRNA-1 (targeting luciferase) or ON-TARGETplus SMARTpool siRNA against Atg7 using the DharmaFECT siRNA transfection reagent #2 (Dharmacon). Twenty-four hours after transfection, cells were collected and re-seeded onto 6-well or 24-well plates and drug-treated for immunoblotting or cytotoxicity analyses, respectively.
Statistical analysis
Data were compared using two-tailed paired Student’s t-test, and P value less than 0.05 was considered significant.
Results
2-DG-induced ER stress/UPR and decreased ATP levels correlate with activation of autophagy in different tumor cell types
Although 2-DG is known as an inhibitor of glycolysis, due to its similarity to mannose, it has also been shown to induce ER stress via interference with N-linked glycosylation [7–9]. To determine whether perturbation of either or both pathways leads to activation of autophagy, ATP levels and ER stress/UPR, markers were assayed in three different human cancer cell lines, i.e., 1420 (pancreatic cancer), MDA-MB-435 (melanoma) [29] and SKBR3 (breast cancer). As can be seen in Fig. 1, in all cell lines ATP levels dropped while the ER stress/UPR markers glucose-regulated protein 78 kD (Grp78) and CHOP increased, when cells were treated with 2-DG in the presence of oxygen. Concomitantly, activation of autophagy was observed as assayed by the conversion of microtubule-associated protein-1 light chain 3B (LC3B) from its unconjugated (LC3B I) to its lipidated form (LC3B II) (Fig. 1). Overall, these data show that 2-DG stimulates autophagy which correlates with ER stress/UPR induction and decreases in ATP levels, suggesting that either or both of these activities of 2-DG are activating autophagy.
2-DG induces ATP reduction, ER stress/UPR and autophagy. 1420 (a), MDA-MB-435 (b) and SKBR3 (c) cells were treated with 2-DG at doses as indicated. Upper panels, intracellular ATP levels were measured after 5 h of drug exposure. Lower panels, immunoblotting was performed to detect levels of Grp78, CHOP and LC3B II after 16 h (1420 and MDA-MB-435) or 24 h (SKBR3) of treatment. β-Actin was used as a loading control. *Bands of LC3B I were not detected by short-time film exposure in 1420 and SKBR3 cells. # P < 0.05 and ## P < 0.01, compared to controls
Mannose reverses 2-DG-induced autophagy and ER stress but not ATP reduction
Recently, our laboratory has shown that 2-DG kills select tumor cell lines, including 1420 cells growing under normal O2 conditions through ER stress/UPR-mediated apoptosis [7]. Furthermore, addition of exogenous mannose was shown to reverse 2-DG-induced ER stress and cytotoxicity. Therefore, mannose was added to 2-DG-treated cells to study autophagy activation when ER stress was abolished. Consistent with our previous work, in 1420 cells 1 mM of mannose reversed 2-DG (4 mM)-induced upregulation of ER stress/UPR markers Grp78 and CHOP (Fig. 2a). Most noteworthy, under these conditions, LC3B II levels reverted back to untreated controls, signifying blockage of autophagy stimulation (Fig. 2a). To compare more carefully the autophagic flux in response to 2-DG and mannose, we conducted experiments in the presence of EST (E64d), a lysosomal protease inhibitor that can block the degradation of LC3B II after the autophagosome fuses with a lysosome. Thus, under these conditions, activation of autophagy is less likely to be underestimated [30, 31]. For each treatment condition, EST increased the accumulation of LC3B II, indicating that this drug was functional in our experimental system. More importantly, essentially the same results were obtained when EST was included in the mannose reversal experiment (Fig. 2a). The ability of mannose to reverse 2-DG-induced autophagy was further confirmed by another widely used method to detect autophagy, namely the appearance of LC3B puncta (Fig. 2b) [30, 31]. In contrast to the reversal of ER stress and autophagy activation, mannose failed to restore 2-DG-induced decreased ATP to untreated levels (Fig. 2c). Moreover, the phosphorylation status of the AMP-activated protein kinase (AMPK) substrate acetyl-CoA carboxylase (ACC) did not change when mannose was present (Fig. 2d), indicating that 2-DG-induced activation of the energy sensing signaling (AMPK pathway) is not affected by exogenous mannose. These data suggest that 2-DG activates autophagy through induction of ER stress and not by depleting ATP.
Mannose reverses 2-DG-induced ER stress and autophagy without affecting ATP depletion. a 1420 cells were treated either by 4 mM of 2-DG, 1 mM of mannose (Man) or both in the presence or absence of 4 μg/ml of EST for 16 h. Immunoblotting was performed to detect levels of Grp78, CHOP and LC3B II. b 1420 cells were treated either by 4 mM of 2-DG or 4 mM of 2-DG with 1 mM of Man for 16 h. Endogenous LC3B were detected by fluorescent microscopy using an anti-LC3B antibody. c Intracellular ATP levels were measured after 1420 cells were treated either by 4 mM of 2-DG, 1 mM of Man or both for 5 h. d 1420 cells were treated similarly as depicted in (a) without EST for 6 h. Immunoblotting was performed to detect phosphorylated (serine 79) and total ACC levels. Representative blots of one of two independent experiments are shown. e MDA-MB-435 cells were treated either by 10 mM of 2-DG, 1 mM of Man or both. Upper panel, intracellular levels of ATP were measured after 5 h of drug exposure. Lower panel , immunoblotting was performed to detect levels of Grp78, CHOP and LC3B II 16 h after treatment. *Bands of LC3B I were not detected by short-time film exposure in 1420 and SKBR3 cells. f MDA-MB-435 cells were treated and analyzed similarly as 1420 cells in (b) except that 2-DG was used at a dose of 10 mM. g SKBR3 cells were treated and analyzed similarly as MDA-MB-435 cells in (e) except that immunoblotting was performed 24 h after drug exposure. For immunoblotting analyses in (a, e, g), β-actin was used as a loading control. NS, not significant compared to 2-DG alone-treated samples
To study whether activation of autophagy by 2-DG through ER stress is a general phenomenon, mannose reversal experiments were extended to two other cancer cell lines. In MDA-MB-435 cells, mannose was found to completely reverse the increased expression of LC3B II as well as Grp78 and CHOP assayed by immunoblotting (Fig. 2e, bottom panel), and to abolish the punctate pattern of LC3B immunofluorescence similarly as in 1420 cells (Fig. 2f). In SKBR3 cells, mannose partially abrogated the upregulation of LC3B II, which correlated with the partial reduction of the ER stress/UPR markers (Fig. 2g, bottom panel). In agreement with the results obtained in 1420 cells, mannose did not affect 2-DG-induced ATP depletion in either of the cell lines (Fig. 2e, g, top panels). Taken together, data from these experiments demonstrate that ER stress/UPR signaling is the predominant mechanism by which 2-DG induces autophagy in multiple human cancer cell lines.
2-DG displays similar kinetics as the ER stress inducer tunicamycin in activating autophagy
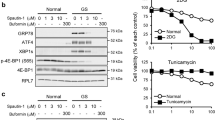
Since tunicamycin induces ER stress similarly to 2-DG in that both of them interfere with N-linked glycosylation [8, 9, 32], the kinetics of this agent to activate autophagy was investigated and compared to that of 2-DG in 1420 cells. Both drugs were found to induce ER stress/UPR in a similar manner during a 24 h period of time, as assessed by the expression of Grp78 and CHOP (Fig. 3a). Treatment with either drug increased peak levels of LC3B II at 16 h with decreasing levels observed at 24 h after drug exposure (Fig. 3a). Similar results of increased accumulation of LC3B II were obtained when experiments were conducted in the presence of EST (Fig. 3b). Interestingly, a sustained upregulation of LC3B II after 24-h (and 48 h, data not shown) exposure to both drugs was observed in the presence of EST when compared to the decrease found when cells were treated in the absence of EST, suggesting that accelerated autophagic degradation is occurring at this time point of drug treatment. Overall, these data illustrate that 2-DG and tunicamycin display similar kinetics in stimulating autophagic activity, further supporting the conclusion that 2-DG-activated autophagy results as a consequence of its induction of ER stress/UPR.
2-DG induces autophagy in a similar kinetics as tunicamycin. 1420 cells were treated with 4 mM of 2-DG or 1 μg/ml of tunicamycin (TM) in the absence (a) or presence (b) of 4 μg/ml EST. At the indicated times, cells were harvested and immunoblotting was performed to detect levels of Grp78, CHOP and LC3B II. β-Actin was used as a loading control. Blots for TM treatment are from one of two independent experiments
Oxamate, which induces ATP depletion but not ER stress, does not activate autophagy
Recent reports indicate that various environmental stresses or drug treatments that can lead to energy depletion induce autophagy [22–28]. This raises the question as to why 2-DG-induced ATP reduction did not appear to contribute to autophagy activation in the cancer cell lines we examined in this study. To better understand the role of ATP depletion in autophagy stimulation, we monitored autophagic activity as described earlier when cells were treated with oxamate, a glycolytic inhibitor that depletes ATP but does not induce ER stress. In 1420 cells, three different doses of oxamate (2, 5 and 10 mg/ml) were studied so that the range of ATP reduction achieved by these doses was similar to that reached by 4 mM of 2-DG (Fig. 4a). As shown in Fig. 4b, at doses of oxamate where ATP was reduced similarly or greater than that by 2-DG, there was no increase in LC3B II while 2-DG (as a positive control) induced an obvious increase in this autophagy marker. In addition, following the kinetics of autophagy activation showed that at 5 mg/ml of oxamate, where ATP levels were reduced to ~50%, and LC3B II levels within 24 h were not significantly affected (Fig. 4c). Consistent with the immunoblotting results of LC3B processing, oxamate at 5 mg/ml did not increase LC3B puncta (Fig. 4d), further confirming that there was no increase in autophagosome formation and autophagy activity when 1420 cells were depleted of ATP by oxamate. Similar experiments were also performed in MDA-MB-435 cells, and essentially the same results were obtained (Fig. 4e–g). Taken together, these data show that ATP depletion induced by oxamate at levels equivalent to that of 2-DG does not induce autophagy in the cell lines used in this study, supporting our conclusion that 2-DG, when lowering ATP moderately in our experimental settings, activates autophagy mainly via ER stress/UPR.
Oxamate induces ATP depletion but not autophagy. a Intracellular ATP levels were measured after 1420 cells were treated by 4 mM of 2-DG or different doses of oxamate (OX) as indicated for 5 h. b 1420 cells were treated by 4 mM of 2-DG or different doses of OX as indicated in the presence or absence of 4 μg/ml of EST for 16 h. Immunoblotting was performed to detect levels of Grp78, CHOP and LC3B II. Representative blots of one of two independent experiments are shown. c 1420 cells were treated with 5 mg/ml of OX in the presence or absence of 4 μg/ml EST. At the indicated times, cells were harvested and immunoblotting was performed to detect levels of LC3B II. d 1420 cells were treated by 5 mg/ml OX for 16 h. Endogenous LC3B was detected by fluorescent microscopy using an anti-LC3B antibody. e MDA-MB-435 cells were treated and analyzed similarly as 1420 cells in (a) except that 2-DG was used at a dose of 10 mM. f MDA-MB-435 cells were treated by 10 mM of 2-DG or different doses of OX as indicated for 16 h. Immunoblotting was performed to detect levels of Grp78, CHOP and LC3B II. *Bands of LC3B I were not detected by short-time film exposure in 1420 cells. Representative blots of one of two independent experiments are shown. g MDA-MB-435 cells were treated and analyzed similarly as 1420 cells in (d) except that 2-DG was used at a dose of 10 mM. For immunoblotting analyses in (b, c, f), β-actin was used as a loading control. # P < 0.05, ## P < 0.01 and ### P < 0.001, compared to controls
2-DG reduces autophagy activity under anaerobic conditions
In hypoxic/anaerobic conditions, cells treated with 2-DG will undergo a more severe drop in ATP levels than when treated under normal O2 tension ([33]; Fig. 5a). Since 2-DG is in a Phase I clinical trial to evaluate its anti-tumor efficacy by targeting hypoxic tumor cells in combination with conventional chemotherapeutics [6], we were prompted to study the autophagic response to 2-DG in cells under hypoxic/anaerobic conditions. However, recent reports have shown that hypoxia itself is able to induce autophagy as well as ER stress/UPR through different mechanisms [34, 35]. Thus, in order to eliminate the complications accompanied by hypoxia with regard to autophagy activation, a previously established chemical model of “hypoxia/anaerobiosis” was used [3]. To our surprise, the 2-DG-induced increase in Grp78 was abolished under anaerobic conditions (cells treated with the mitochondrial ATP synthase inhibitor, oligomycin), and in fact, even below the basal levels of cells left untreated (Fig. 5b). Interestingly, the expression of LC3B II displayed a similar decrease in response to 2-DG under anaerobic conditions (Fig. 5b). This decrease in LC3B II reflected a slowing-down of autophagic flux rather than an acceleration of autophagic proteolysis since blockage of lysosomal degradation by EST did not restore LC3B II to basal levels (Fig. 5b). To better understand this reduction of autophagy in response to 2-DG under anaerobic conditions, LC3B II changes were monitored over 24 h. Whereas no change was detected after 8 h of treatment, LC3B II levels began to decrease at 16 h, and continued to diminish during the remainder of the experiment (Fig. 5c). Moreover, the LC3B immunofluorescence data further showed that there was a slight reduction of autophagosome formation in 1420 cells treated with 2-DG in our chemical model of “hypoxia” (Fig. 5d). Similar to the results obtained in 1420 cells, 2-DG also induced a severe ATP drop, and reduced LC3B II as well as Grp78 under anaerobic conditions in MDA-MB-435 cells (Fig. 5e, f). Overall, these data further reveal a close correlation between ER stress/UPR signaling and autophagy activation, and show that in contrast to the stimulation of autophagy by 2-DG in cells grown aerobically, autophagy activity was diminished by this glucose analog under anaerobic conditions.
Under anaerobic conditions, 2-DG severely depletes ATP and down-regulates autophagy. a Intracellular ATP levels were measured after 1420 cells were treated either by 4 mM of 2-DG, 0.1 μg/ml of oligomycin (OM) or both for 5 h. b 1420 cells were treated either by 4 mM of 2-DG, 0.1 μg/ml of OM or both in the presence or absence of 4 μg/ml of EST for 16 h. Immunoblotting was performed to detect levels of Grp78 and LC3B II. Representative blots of one of two independent experiments are shown. c 1420 cells were treated by 4 mM of 2-DG in combination with 0.1 μg/ml of OM in the presence or absence of 4 μg/ml EST. At the indicated times, cells were harvested and immunoblotting was performed to detect levels of LC3B II. d 1420 cells were treated by 4 mM of 2-DG in combination with 0.1 μg/ml of OM for 16 h. Endogenous LC3B were detected by fluorescent microscopy using an anti-LC3B antibody. e MDA-MB-435 cells were treated and analyzed similarly as 1420 cells in (a) except that 2-DG was used at a dose of 10 mM. f MDA-MB-435 cells were treated either by 4 mM of 2-DG, 0.1 μg/ml of OM or both for 16 h. Immunoblotting was performed to detect levels of Grp78 and LC3B II. *Bands of LC3B I were not detected by short-time film exposure in 1420 cells. For immunoblotting analyses in (b, c, f), β-actin was used as a loading control. ## P < 0.01, compared to 2-DG alone-treated samples
Autophagy promotes cancer cell survival by reducing 2-DG-induced ER stress
2-DG has shown anti-tumor activity both in vitro and in vivo [36] and has entered a Phase I clinical trial. In this regard, we next investigated the role autophagy plays in 2-DG-elicited cytotoxicity in cancer cells. In agreement with previous reports [37, 38], we found that autophagy protected cells from 2-DG-induced cell death in various cancer cell lines. On one hand, 3-MA, an autophagy inhibitor by blocking the class III phosphatidylinositol 3-kinase (PI3K III) [39], increased 2-DG-induced cell death in 1420 cells and sensitized MDA-MB-435 cells to 2-DG treatment (Fig. 6a, b). On the other hand, the mammalian target of rapamycin (mTOR) inhibitor and autophagy promoter rapamycin reduced cell death in both 1420 and SKBR3 cells treated with 2-DG (Fig. 6c, d).
Autophagy protects cancer cells from 2-DG-induced cytotoxicity through relieving ER stress. a, b 1420 (a) and MDA-MB-435 (b) cells were treated with the indicated doses of 2-DG in the presence or absence of 10 mM of 3-methyladenine (3-MA) for 48 h. The percentages of dead cells were analyzed based on trypan blue exclusion. c, d 1420 (c) and SKBR3 (d) cells were treated with the indicated doses of 2-DG in the presence or absence of 0.1 μg/ml of rapamycin (Rap) for 48 h (SKBR3) or 72 h (1420). The percentages of dead cells were analyzed based on trypan blue exclusion. e 1420 cells were transfected with either a siRNA targeting luciferase (siLuc, negative control) or a pool of siRNAs against Atg7 (siAtg7) at 50 nM. Forty-eight hours post-transfection, cells were treated with different doses of 2-DG, and 16 h later, harvested and immunoblotted for expression levels of Atg7, LC3B II, Grp78, CHOP and cleaved caspase 3. β-Actin was used as a loading control. Representative blots of one of two independent experiments are shown. f 1420 cells were transfected as depicted above. Forty-eight hours post-transfection, cells were treated with different doses of 2-DG for another 48 h. The percentages of dead cells were then analyzed based on trypan blue exclusion. Representative results of one of two independent experiments are shown. # P < 0.05 and ## P < 0.01, compared to the corresponding 2-DG alone-treated samples (a, b, c, d) or 2-DG-treated cells transfected with siLuc (f)
Previously, we have reported that 2-DG causes cell death through ER stress/UPR-mediated apoptosis in selected cancer cell lines under normoxia, including 1420 cells [7]. To investigate whether autophagy reduces 2-DG-induced cytotoxicity through modulating ER stress, we examined autophagy, ER stress/UPR and apoptosis markers in response to 2-DG treatment in 1420 cells where the expression of the autophagy-related gene 7 (Atg7), which is indispensable for this process, was specifically knocked down by short interfering RNA (siRNA). As shown in Fig. 6e, siRNA against Atg7 successfully reduced the protein levels of Atg7 compared to that against luciferase (siLuc), which was used as a siRNA control in this set of experiments. Knocking down of Atg7 attenuated the induction of LC3B II by 2-DG, which correlates with the increase in the expression of the ER stress/UPR markers Grp78 and CHOP (at 8 mM of 2-DG) when assayed at 16 h of drug treatment (Fig. 6f). Additionally, at the same time point, levels of the apoptosis marker cleaved caspase 3 were significantly increased above those in cells transfected with siLuc (Fig. 6f), which corresponds with increased cell death assayed at 48 h of 2-DG exposure (Fig. 6e). These observations support the findings from other groups that autophagy acts as a survival mechanism against ER stress-induced toxicity in both yeast and mammalian cells [13, 14, 18, 40, 41]. In summary, our data presented here demonstrate that autophagy protects cancer cells from 2-DG-induced cytotoxicity and indicate that this is likely achieved through relieving 2-DG-elicited ER stress.
Discussion
Although data from our laboratory, as well others [37, 38] demonstrate that autophagy, an evolutionarily conserved intracellular bulk degradation process, responds to 2-DG treatment, the precise mechanism(s) by which 2-DG-induced autophagy occurs is not fully understood. Here, we find that autophagy activation in response to 2-DG is accompanied by ER stress and UPR signaling as well as decreased cellular ATP levels (Fig. 1). Previously, White’s [37] and Yang’s [38] groups independently reported that 2-DG activates autophagy in cancer cells; and in the latter study, the authors suggested that the activation of eukaryotic elongation factor-2 kinase (eEF-2K) is responsible for the induction of autophagy resulting from energy depletion (lowered ATP) due to 2-DG treatment. However, in our experimental systems, addition of exogenous mannose to 2-DG-treated cells, which we previously reported reduces ER stress [7], abolishes autophagy but does not restore 2-DG-induced ATP depletion (Fig. 2). Furthermore, results as shown in Figs. 3 and 4 demonstrate that in the cell lines studied, 2-DG induces autophagy in a similar manner as the classical ER stress inducer tunicamycin. In contrast, oxamate, another glycolytic inhibitor which does not disrupt ER homeostasis but reduces ATP levels does not activate autophagy. Thus, our results point to ER stress, and not lowered ATP, as the predominant mechanism by which 2-DG induces autophagy.
The discrepancies between the results from Yang’s group and ours as to how 2-DG stimulates autophagy might be explained by recent results which showed that ER stress induced by tunicamycin stimulates eEF-2K [42]. In a subsequent study, the same group reported that ER stress-stimulated eEF-2K participates in autophagy activation [43]. Thus, activation of eEF-2K could be a downstream event in response to ER stress to induce autophagy independently of ATP depletion. In fact, in one of the cell lines (T98G) used in Yang’s study, we find that mannose is also able to reverse 2-DG-induced autophagy as well as ER stress (data not shown), which further supports our conclusion that ER stress/UPR is the major activator of autophagy in response to 2-DG.
Under normal O2 conditions, the moderate reduction of ATP by 2-DG (~50% at 5 h treatment) does not appear to play a significant role in autophagy activation. Since 2-DG is currently under clinical trial directed at selectively targeting the hypoxic malignant cell population in solid tumors [6], we addressed the question of how autophagy responds to 2-DG when cells are grown under anaerobic conditions (cells treated with oligomycin) [3]. As expected, ATP levels are severely reduced, but to our surprise, in contrast to the upregulation of autophagic activity in cells grown aerobically, 2-DG decreases autophagy even below basal levels when cells are grown anaerobically (Fig. 5). Under the same experimental conditions, reduction of autophagy is accompanied by a similar pattern of decrease in the ER stress/UPR marker Grp78 (Fig. 5). Moreover, under hypoxic conditions (0.1%), reduced levels of LC3B II and Grp78 were also observed when cells were similarly treated with 2-DG (data not shown). Thus, it appears that a critical level of intracellular ATP may be necessary for either UPR and/or autophagy to take place. It remains unclear whether severely lowered ATP levels interfere with the activation of UPR which in turn leads to reduction of autophagy or whether lowered ATP levels directly inhibit the autophagic activity itself [44–47]. Further experiments are ongoing in our laboratory to distinguish between these possibilities.
It has been previously shown that autophagy can act either as a pro- or anti-survival mechanism [20, 48, 49]. Here, our observations that rapamycin, which is known to stimulate autophagy, alleviates 2-DG toxicity (Fig. 6c, d), whereas 3-MA which blocks autophagy aggravates it (Fig. 6a, b), suggest that autophagy acts as a pro-survival adaptive response to 2-DG. These findings are in agreement with two recent reports [37, 38] showing that inhibition of autophagy increases the anti-tumor effect of 2-DG in vitro. In one of these studies [38], 2-DG-induced autophagy was inhibited with siRNA against eEF2-K and ATP levels were lower than in cells treated with non-targeting siRNA. Thus, the authors concluded that grater ATP depletion via autophagy blockade leads to increased cell death. However, previous findings from our laboratory have demonstrated that moderate energy depletion achieved by 2-DG under normoxia is not sufficient to kill most tumor cells and the mechanism by which 2-DG kills a subset of cancer cells in the presence of O2 is via ER stress/UPR-mediated apoptosis [7]. Moreover, our results, which show that inhibiting 2-DG-induced autophagy through siRNA targeting Atg7 increases the levels of ER stress/UPR markers as well as apoptosis (Fig. 6e), are consistent with reports from other groups demonstrating that autophagy promotes cell survival in response to ER stress [13, 14, 18, 40, 41]. These results further support our findings with 3-MA and rapamycin, that autophagy plays a protective role against 2-DG-induced cytotoxicity, probably through relieving ER stress.
The identification of ER stress markers (glucose-regulated proteins) originated from studies in which cells were starved of glucose [50]. Thus, since most solid tumors undergo some level of glucose starvation, our studies with 2-DG should have relevance to ER stress-induced autophagy in carcinomas. It will be interesting to contrast our results with 2-DG with those in cells starved of glucose, which should contribute to a better understanding of the interactions between microenvironmental conditions, autophagy activation and tumor cell survival. In addition, our findings may have therapeutic implications for improving the anti-tumor performance of 2-DG by combining it with autophagy inhibitors. Therefore, further elucidation of the participants in transmitting signals from 2-DG- and/or glucose deprivation-induced ER stress to autophagy appears warranted with the potential for identifying novel targets for selectively blocking autophagy.
Abbreviations
- 2-DG:
-
2-Deoxy-d-glucose
- 3-MA:
-
3-Methyladenine
- Atg7:
-
Autophagy-related gene 7
- CHOP:
-
C/EBP homologous protein
- eEF-2K:
-
Eukaryotic elongation factor-2 kinase
- ER:
-
Endoplasmic reticulum
- Grp78:
-
Glucose-regulated protein 78 kD
- LC3B:
-
Microtubule-associated protein-1 light chain 3B
- UPR:
-
Unfolded protein response
References
Wick AN, Drury DR, Nakada HI, Wolfe JB (1957) Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem 224:963–969
Chen W, Gueron M (1992) The inhibition of bovine heart hexokinase by 2-deoxy-d-glucose-6-phosphate: characterization by 31P NMR and metabolic implications. Biochimie 74:867–873
Liu H, Hu YP, Savaraj N, Priebe W, Lampidis TJ (2001) Hypersensitization of tumor cells to glycolytic inhibitors. Biochemistry 40:5542–5547
Liu H, Savaraj N, Priebe W, Lampidis TJ (2002) Hypoxia increases tumor cell sensitivity to glycolytic inhibitors: a strategy for solid tumor therapy (model C). Biochem Pharmacol 64:1745–1751
Maher JC, Krishan A, Lampidis TJ (2004) Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-d-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol 53:116–122
Raez LE, Rosenblatt J, Schlesselman J et al (2005) Combining glycolytic inhibitors with chemotherapy: phase I trial of 2-deoxyglucose and docetaxel in patients with solid tumors [abstract no: 3190]. In: American Society of Clinical Oncology Annual Meeting, May 13–17 Orlando, FL
Kurtoglu M, Gao N, Shang J et al (2007) Under normoxia, 2-deoxy-d-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol Cancer Ther 6:3049–3058
Datema R, Schwarz RT (1978) Formation of 2-deoxyglucose-containing lipid-linked oligosaccharides Interference with glycosylation of glycoproteins. Eur J Biochem 90:505–516
Datema R, Schwarz RT (1979) Interference with glycosylation of glycoproteins. Inhibition of formation of lipid-linked oligosaccharides in vivo. Biochem J 184:113–123
Kurtoglu M, Maher JC, Lampidis TJ (2007) Differential toxic mechanisms of 2-deoxy-d-glucose versus 2-fluorodeoxy-d-glucose in hypoxic and normoxic tumor cells. Antioxid Redox Signal 9:1383–1390
Szegezdi E, Fitzgerald U, Samali A (2003) Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci 1010:186–194
Schroder M, Kaufman RJ (2005) ER stress and the unfolded protein response. Mutat Res 569:29–63
Bernales S, McDonald KL, Walter P (2006) Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4:e423
Ogata M, Hino S, Saito A et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231
Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281:30299–30304
Hoyer-Hansen M, Jaattela M (2007) Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 14:1576–1582
Ding WX, Ni HM, Gao W et al (2007) Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem 282:4702–4710
Kouroku Y, Fujita E, Tanida I et al (2007) ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ 14:230–239
Sakaki K, Wu J, Kaufman RJ (2008) Protein kinase c theta is required for autophagy in response to stress in the endoplasmic reticulum. J Biol Chem 283:15370–15380
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6:463–477
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93
Kuma A, Hatano M, Matsui M et al (2004) The role of autophagy during the early neonatal starvation period. Nature 432:1032–1036
Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB (2005) Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120:237–248
Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, Meijer AJ (2006) AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem 281:34870–34879
Xu ZX, Liang J, Haridas V, Gaikwad A, Connolly FP, Mills GB, Gutterman JU (2007) A plant triterpenoid, avicin D, induces autophagy by activation of AMP-activated protein kinase. Cell Death Differ 14:1948–1957
Liang J, Shao SH, Xu ZX et al (2007) The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 9:218–224
Papandreou I, Lim AL, Laderoute K, Denko NC (2008) Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ 15:1572–1581
Wei S, Kulp SK, Chen CS (2010) Energy restriction as an antitumor target of thiazolidinediones. J Biol Chem 285:9780–9791
Christgen M, Lehmann U (2007) MDA-MB-435: the questionable use of a melanoma cell line as a model for human breast cancer is ongoing. Cancer Biol Ther 6:1355–1357
Klionsky DJ, Abeliovich H, Agostinis P et al (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4:151–175
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326
Duksin D, Mahoney WC (1982) Relationship of the structure and biological activity of the natural homologues of tunicamycin. J Biol Chem 257:3105–3109
Maher JC, Wangpaichitr M, Savaraj N, Kurtoglu M, Lampidis TJ (2007) Hypoxia-inducible factor-1 confers resistance to the glycolytic inhibitor 2-deoxy-d-glucose. Mol Cancer Ther 6:732–741
Wouters BG, Koritzinsky M (2008) Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer 8:851–864
Mazure NM, Pouyssegur J (2009) Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol 22:177–180
Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR, Lampidis TJ (2004) 2-deoxy-d-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res 64:31–34
DiPaola RS, Dvorzhinski D, Thalasila A et al (2008) Therapeutic starvation and autophagy in prostate cancer: a new paradigm for targeting metabolism in cancer therapy. Prostate 68:1743–1752
Wu H, Zhu H, Liu DX, Niu TK, Ren X, Patel R, Hait WN, Yang JM (2009) Silencing of elongation factor-2 kinase potentiates the effect of 2-deoxy-d-glucose against human glioma cells through blunting of autophagy. Cancer Res 69:2453–2460
Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P (2000) Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem 275:992–998
Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, Yin XM (2007) Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol 171:513–524
Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, Hayashi YK, Momoi T (2007) Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum Mol Genet 16:618–629
Boyce M, Py BF, Ryazanov AG, Minden JS, Long K, Ma D, Yuan J (2008) A pharmacoproteomic approach implicates eukaryotic elongation factor 2 kinase in ER stress-induced cell death. Cell Death Differ 15:589–599
Py BF, Boyce M, Yuan J (2009) A critical role of eEF-2K in mediating autophagy in response to multiple cellular stresses. Autophagy 5:393–396
Plomp PJ, Wolvetang EJ, Groen AK, Meijer AJ, Gordon PB, Seglen PO (1987) Energy dependence of autophagic protein degradation in isolated rat hepatocytes. Eur J Biochem 164:197–203
Plomp PJ, Gordon PB, Meijer AJ, Hoyvik H, Seglen PO (1989) Energy dependence of different steps in the autophagic-lysosomal pathway. J Biol Chem 264:6699–6704
Schellens JP, Meijer AJ (1991) Energy depletion and autophagy. Cytochemical and biochemical studies in isolated rat hepatocytes. Histochem J 23:460–466
Meijer AJ (2009) Autophagy research: lessons from metabolism. Autophagy 5:3–5
Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ (2007) Potential therapeutic applications of autophagy. Nat Rev Drug Discov 6:304–312
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132:27–42
Lee AS (2001) The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci 26:504–510
Acknowledgments
This work is supported by the National Cancer Institute grant# CA37109 to T.J.L. and V.A. Research Merit Award to N.S.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xi, H., Kurtoglu, M., Liu, H. et al. 2-Deoxy-d-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother Pharmacol 67, 899–910 (2011). https://doi.org/10.1007/s00280-010-1391-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1391-0