Abstract
Purpose
Noscapine (NOS) is a unique class of tubulin-binding anticancer agents. Their potential usefulness as anticancer drugs is however limited by the poor bioavailability, thus necessitating administration of a higher dose regime in the range of 300–600 mg/kg for tumor growth inhibition. To augment bioavailability, we prepared an inclusion complex of NOS in β-cyclodextrin (β-CD) and evaluated its physico-chemical characteristics.
Method and results
Our phase-solubility analysis shows a 1:1-complexation (K c ~0.454 mM−1) of NOS with β-CD that offers better dissolution properties. We confirmed complex formation in solid state by differential scanning calorimetry, powder X-ray diffractometry, Fourier-transform infrared spectroscopy, 1H nuclear magnetic resonance spectroscopy, rotating frame Overhauser enhancement spectroscopy and by molecular modeling methods. Based upon theoretical calculations in gas phase, we propose O–CH2–O– in orientation of NOS in the β-CD cavity. The thermal behavior data also provides complementary evidences of complex formation. The pharmacokinetic studies showed a 1.87-fold increase in bioavailability of NOS upon complexation in the β-CD inclusion complex state as compared to free NOS. Furthermore, the complex retains the anticancer attributes of NOS.
Conclusion
Our studies propose for the first time a stable NOS–β-CD inclusion complex as an effective approach to enhance the solubility and bioavailability of NOS for anticancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Noscapine (NOS), an antitussive opium alkaloid (Fig. 1a) has been recently discovered as a tubulin-binding anticancer drug that causes cell cycle arrest and induces apoptosis in cancer cells both in vitro as well as in vivo [1–4]. A unique edge of NOS is that it can be administered orally and has no detectable toxicity. NOS is a weak base (pKa ~7.8) with 42.22% absolute bioavailability and a half-life of 1.47-h by oral route. This necessitates relatively high doses of drug (ED50 300 mg/kg bwt) for induction of anticancer activity [5, 6]. However, the development of oral controlled-release anticancer formulations of NOS is severely hampered due to short biological half-life (<2 h), large dose, poor absorption, low dissolution or aqueous solubility and extensive first pass metabolism [6, 7]. Rather than using co-solvents to enhance the solubility of the drug, which can be irritant or toxic [8], supramolecular hosts, such as cyclodextrins (CDs) could be an attractive option to overcome these limitations. CDs, cyclic oligosaccharides have potentials to alter physical, chemical and biological properties of guest molecules through the formation of inclusion complexes. The α-, β-, γ -CDs are the most common natural CDs consisting of six, seven and eight d-glucopyranose residues, respectively, linked by α-1,4 glycosidic linkage into a macrocycle [9]. Among the above-mentioned CDs, β-CD, with an inner cavity diameter of 6.0–6.5 Å and a depth of 7.9 Å, is the most widely used complexing agent (Fig. 1b). The CD molecule resembles truncated cones with the secondary hydroxyl groups located at the wider edge of the ring and the primary groups on the narrow edge. Methine hydrogens are directed to the inner part of the ring resulting in a hydrophobic cavity along with a hydrophilic character outside the ring [10]. Hence, CDs are soluble in water (β-CD; 1.85 g/100 ml at 25°C) and at the same time may accommodate a broad range of hydrophobic molecules of appropriate dimensions and shapes with formation of inclusion complexes. These are commonly used as molecular inclusion complexing agents to increase the aqueous solubility, bioavailability and stability of poorly water-soluble drugs [11, 12]. Therefore, CDs and its derivatives are valuable for engineering the novel host–guest molecular complexes. Hence, in the present investigation, complexation of NOS with β-CD was attempted. Further, the dissolution property and its bioavailability were evaluated by pharmacokinetic studies. The interaction of NOS with hydrophobic domain of β-CD was assessed by the application of molecular modeling using semiempirical methods.
Materials and methods
Chemicals
NOS [(3S)-6,7-Dimethoxy-3-((5R)-5,6,7,8-tetrahydro-4-methoxy-6-methyl-1,3-dioxolo (4,5-g) isoquinolin -5-yl)- 1(3H)-isobenzofuranone] was purchased from Aldrich Chemicals, USA. β-CD (Mw ~1,135) was a kind gift from University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh, India. Acetonitrile and methanol (HPLC grade) were purchased from Merck, India. All other chemicals used were of A.R. grade. All solutions were prepared using ultra pure water (MILLI Q).
Analytical estimation of noscapine
NOS is soluble in 0.02 M hydrochloric acid and thus used for quantification of NOS in pharmaceutical dosage forms [13]. In the present investigation, simple UV spectroscopy method was used for the determination of NOS. The standard regression equation was prepared as follows. 10.0 mg of standard NOS, dried to constant weight, was dissolved in 0.02 M hydrochloric acid to make 100 ml stock solution, of which different volumes were used to prepare the working solution. NOS showed a maximum wavelength at 311.2 nm. The value of A 311.2 of the standard NOS solutions were used to produce a plot of A 311.2 versus the concentration of NOS, allowing the following standard regression equation in the range of C = 10–100 μg/ml to be written as Eq. 1.
Phase-solubility analysis
Phase-solubility assay for determination of stoichiometry of NOS–β-CD binary system was performed according to the method of Higuchi and Connors [14]. An excess amount of NOS (20 mg) was added to the β-CD (Mw ~1,135), β-CD dissolved in phosphate buffer saline, PBS (10 ml; pH 7.4) at various concentrations (2–17 mM). The concentration range of β-CD was set based on the maximum solubility of β-CD in water at 37°C (1.85 g/100 ml ≈ 16 mM/L at 25°C). The contents were stirred for 72 h at 37 ± 1°C. After equilibrium, the samples were filtered (0.22-μm membrane filter) and absorbance was read at 311.2 nm (Shimadzu 1601 UV/VIS spectrophotometer, Shimadzu Corp, Kyoto, Japan). The apparent stability constant was calculated from the slope of the phase-solubility diagram using Eq. 2.
where So is the solubility of drug in the absence of β-CD.
Preparation of inclusion complexes
The physical mixture (PM) of NOS and β-CD in 1:1 M ratio was obtained by mixing individual component that had previously been passed through # 100 μm sieve. Freeze drying method [15] with slight modification was used to synthesize the inclusion complex by dissolving NOS and β-CD in water in 1:1 mM and pH was adjusted up to 4.5 with 0.02 M hydrochloric acid. The resultant solution was stirred for 24 h in orbit shaker at room temperature. Subsequently, the solution was lyophilized and sample obtained was collected and passed through # 100 μm sieve.
Physico-chemical characterization of solid binary systems
Fourier-transform infrared spectra (FTIR)
Fourier-transform infrared spectra (FTIR) were recorded for NOS, β-CD, PM of NOS and β-CD (1:1) as well as of NOS–β-CD inclusion complex using Spectrum BX (Perkin Elmer) infrared spectrophotometer. Samples were prepared in KBr disk (2 mg sample/200 mg KBr) with a hydrostatic press at a force of 40 psi for 4 min. The scanning range was 450–4,000 cm−1 and the resolution was 4 cm−1.
Differential scanning calorimetry (DSC)
Thermal behavior of NOS, β-CD, PM of NOS and β-CD and NOS–β-CD inclusion complex was examined using a differential scanning calorimetry (DSC) Q 10V 8.1 Build 261(Universal V 3.9A TA Instruments) thermal analyzer. Argon was used as carrier gas and the DSC analysis was carried out at a heating rate of 10°C/min and an argon flow rate of 35 cc/min. The sample size was 5 mg and the observations were recorded at the temperature range of 0 to 300°C.
Powder X-ray diffraction (PXRD)
Powder X-ray diffraction (PXRD) was performed using a RIGAKU, Rotaflex, RV 200 (Rigaku Corporation, Japan) with Ni-filtered, Cu K α-radiation, at voltage of 60 kV and current of 50 mA. The scanning rate employed was 1°/min over the 10–60°C diffraction angle (2θ) range. The PXRD patterns of NOS crystals, β-CD, PM of NOS and β-CD as well as NOS–β-CD inclusion complex were recorded.
Molecular modeling: geometry optimization and calculation of the transition energies
Molecular modeling study was carried out to understand the inclusion orientation of NOS in β-CD. The inclusion complex was emulated by entering the guest molecule (NOS) from one end of the β-CD molecule and then letting it pass through the host molecule by steps. Two inclusion orientations were taken into consideration: the first one with O–CH2–O– pointing towards the β-CD cavity (both the hydrogen atoms in) and the other with the H3CO–C6H4–OCH3 group pointing towards the cavity. These two orientations are formed to be the representative orientations, though many alternative confirmations are possible. The minimum energy structure was calculated in gas phase using semiempirical AM1 (Austin model 1) [16] and PM3 (parameterized model number 3) [17] methods implemented in Gaussian 03 [18] software. Complete optimizations have been performed (in gas phase) using default values for the positional deviation of atoms and forces as implements in Gaussian 03 software.
Nuclear magnetic resonance (NMR) measurements
All the 1H NMR spectra were recorded with BRUKER AVANCE II 400 MHz spectrometer at 294.3 K, and the data processing was performed with TOP SPIN 1.3 standard software. All one-dimensional spectra obtained were deconvoluted with Nuts NMR data processing software (Acorn NMR Inc.). The 1H NMR spectrum of NOS in D2O could not be performed due to its very low aqueous solubility; therefore 1H NMR signal assignments for NOS were performed in DMSO-d6 at 0.04 mg/ml, whereas β-CD and inclusion complex (NOS–β-CD; 1:1) in D2O solution containing 5.67 mg/ml (0.5 mM) of β-CD and 0.04 mg/ml of NOS–β-CD inclusion complex, respectively. The chemical shift (δ) was referenced to the internal signal of TMS (0.01%). 1H NMR chemical shifts (δ ppm) caused upon complexation were measured to confirm the inclusion of NOS in β-CD.
The 400-MHz ROESY spectroscopy (phase-sensitive rotating frame Overhauser enhancement spectroscopy) for a solution containing NOS–β-CD inclusion complex (0.04 mg/ml in D2O) was performed with the BRUKER standard pulse sequences; data consisted of 8 transients collected over 2,048 complex points. A mixing time of 700 ms, a repetition delay of 1.0 s, and a 900-pulse width of 10 μs were used. The ROESY data set was processed by applying an exponential function in both dimensions and zero filling to 2,048 × 2,048 real data points prior to the Fourier transformation. Small cross peaks on the ROESY spectra were neglected, because their magnitude was close to that of noise. The volume of a ROESY cross peak was calculated by summation of spectrum intensities with a certain region around the cross peak and slightly depended on the region of integration, the peak overlap and the signal-to-noise ratio.
Determination of solubility
An excess amount of NOS was added to 10 ml of PBS (pH 7.4) and shaken vigorously for 24 h in an orbit shaker at 37 ± 0.5°C. Subsequently, the contents were centrifuged and supernatant liquid was filtered through 0.22-μm membrane filter (MDI, India) and analyzed in UV-Visible Spectrophotometer (Shimadzu, Japan) at 311.2 nm. The above procedure was repeated for the NOS–β-CD inclusion complex. The procedure was repeated thrice for NOS and NOS–β-CD inclusion complex and a mean of three observations were calculated.
Dissolution study
Dissolution studies were conducted for pure NOS, PM of NOS and β-CD (1:1), NOS–β-CD inclusion complex (1:1) and for NOS hydrochloride using USP paddle type dissolution test apparatus at 37 ± 1°C at 100 rpm. The dissolution medium used was 900 ml of simulated gastric fluid (SGF; pH 1.2) without pepsin and PBS of pH 7.4. NOS, NOS hydrochloride, PM of NOS and β-CD as well as NOS–β-CD inclusion complex were filled in separate hard gelatin capsule shells so as to contain 50 mg NOS per capsule. Sampling was performed after 5, 15, 30, 45, 60, 90 and 120 min and equivalent amount of fresh dissolution medium was added to mimic infinite sink conditions. The NOS content was determined spectrophotometrically at 311.2 nm (Shimadzu, Japan). Data were expressed as % of NOS released for each sample. All studies were carried out in triplicate.
Pharmacokinetics
Male Swiss albino mice (NICD, New Delhi, India) were used. The animals were housed in controlled environment (23 ± 1°C, 50 ± 5% humidity) and allowed access to food and water ad libitum. The room lights were kept on between 8.00 a.m. and 8.00 p.m. All experiments were performed in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animal (CPCSEA), Ministry of Culture, Government of India. The Institutional Animal Ethics Committee approved the study. In order to assess the potential of β-CD to enhance the oral bioavailability of NOS, we have developed a high performance liquid chromatography (HPLC) method for determination of NOS in plasma samples in our laboratory [6]. Bioavailability of NOS and NOS–β-CD inclusion complex was evaluated following this method in male Swiss albino mice.
HPLC system consisted of Shimadzu instruments equipped with a Shimadzu LC-10 A pumps and an SPD-10Avp (diode array) detector. The analytical column used was LiChrospher @ 60 RP select-B (C8) reversed-phase column (4 × 125 mm) packed with 5-μm particles. The column temperature was equivalent to room temperature (25°C). The mobile phase consisted of ammonium acetate (Merck): acetonitrile (Merck, HPLC grade) (65:35). 20 mM ammonium acetate solution was adjusted up to pH 4.5 using glacial acetic acid. The solution was filtered and degassed by vacuum filtration through a 0.22-μm membrane filter (MDI, India) before use and was freshly prepared for each run. The flow rate of the mobile phase was adjusted to 1 ml/min. The wavelength of detection was 232 nm and the detector was set at 0.005 absorbance unit, full scale. Pharmacokinetic study was carried out on male mice: 60 male mice were randomly divided into 20 groups of 3 animals each corresponding to the time points of blood collection. Although this gives us three mice per time point for analysis, to ensure that our systemic error is acceptable we had analyzed each sample in triplicates and seen no variation (at 95% confidence level). NOS and NOS equivalent to 150 mg/kg in β-CD inclusion complex were administered orally by gavages. Plasma levels of NOS were monitored for 24 h after oral administration of NOS and NOS–β-CD inclusion complex. Samples were collected at 0, 0.083, 0.25, 0.5, 1, 2, 4, 6, 8 and 24 h in both groups. Approximately, 750 μl of blood was collected from the retro-orbital vein into polypropylene microcentrifuge tubes containing 25 μl of 20 mM sodium citrate. The blood samples were centrifuged at 4,600 rpm for 15 min at 4°C and the plasma was separated. Plasma samples were stored at −80°C until analyzed. Plasma concentration data was analyzed with standard non-compartmental methods using WINNONLIN software version 4.1. Composite plasma concentration–time profiles were constructed for male mice. The following pharmacokinetic parameters were assessed: t 1/2 (h), t max (h), C max (μg/ml), AUClast (h μg/ml), AUCinf (h μg/ml). The area under the curve (AUClast) was calculated by the linear trapezoidal rule up to the last sampling point with detectable levels with extrapolation to infinity (AUCinf) by the Eq. 3.
where k e represents the terminal disposition rate constant. k e was calculated from the slope of the data points in the final log linear part of the drug–concentration–time curve by weighted least square linear regression analysis. The terminal disposition half-life (t 1/2) value was calculated using Eq. 4.
Maximum plasma concentration (C max) and the time to maximum concentration (t max) following oral administration were estimated by recording directly from experimental observations. The bioavailability was calculated using the formula:
Stability study
NOS–β-CD inclusion complexes were stored at 40°C/75% RH for 3 months and the formulations were observed for changes in drug content.
Statistical analysis
The results are expressed, as the mean ± S.D for n ≥ 3. Statistical significance was determined with a one-way analysis of variance and t test. P < 0.05 was taken as a significant level of difference.
Results
Phase-solubility analysis
Figure 2 represents the phase-solubility diagram for NOS–β-CD binary system. Our results show a linear increase in solubility of NOS with increasing concentration of β-CD. The aqueous solubility of NOS increased linearly (slope 0.005) as a function of β-CD concentration. The stability constant (K c ) of NOS and β-CD complex (1:1) was calculated as 0.454 mM−1 from the linear plot of the phase-solubility diagram.
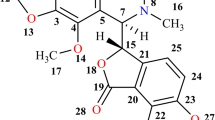
Molecular structures of (a) NOS, (b) NOS–β-CD inclusion complex (O–CH2–O)-in orientation, (c) NOS–β-CD inclusion complex (CH3O–C6H4–OCH3)-in orientation and (d) ROESY of NOS–β-CD inclusion complex (1:1). Results indicated that O–CH2–O– in orientation is more stable than H3CO–C6H4–OCH3– in orientation, indicating the insertion of O–CH2–O– moiety of NOS in β-CD hydrophobic cavity. The orientation of protons in ROESY recorded in D2O supports the results of molecular modeling showing the cross peak of O–CH2–O– protons, indicated the insertion of –O–CH2–O– protons of NOS in β-CD cavity
Characterization of solid complexes
We characterized solid inclusion complexes by various spectroscopy techniques. FTIR spectra were recorded to analyze the structure of new chemical linkage formed during the inclusion of NOS in β-CD. The FTIR spectrum peaks of NOS, β-CD, PM and the inclusion complex are shown in Table 1. The FTIR spectrum of NOS shows a characteristic peak at 1,755 cm−1, indicating the presence of intact lactone ring in the NOS molecule and also the overall stability of molecule. Other peaks of NOS observed are 2,985 cm−1 for C=O stretching vibrations, 1,425 and 1,388 cm−1 for N–CH3 bending vibrations and 2,944, 2,882, 2,842, 2,798 cm−1 due to various –OCH3/CH3 groups. The spectrum of pure β-CD reveals the vibration of free –OH groups between 3,300 and 3,500 and 2,925 cm−1 for C–H stretching as in CH2 and for CH3. However, insertion by means of inclusion complexation in 1:1 completely masked the characteristic peak such as 2,798 cm−1 indicating the absence of OCH3/OCH2 group or insertion of methoxy group in the β-CD cavity. Moreover, peak of lactone ring present in NOS (1,755 cm−1) was also shifted to 1,762 cm−1 in the inclusion complex. But this shift is not significant.
We ascertained the formation of a 1:1 inclusion complex in solid state using DSC (Fig. 4a–e). The DSC curve of pure NOS showed a sharp endotherm near 177.82°C that is indicative of its melting temperature (174–176°C). The thermograms of all CDs (i.e. α-, β-, γ-CDs) show a broad endothermal effect ranging from 40 to 150°C (89.24°C) due to the dehydration process. The DSC thermogram of β-CD was characterized by a small endothermal peak at about 220°C, which may be attributed to a reverse transformation of β-CD. The DSC pattern of NOS–β-CD physical mixture reveals the presence of peaks of both pure compounds except with the difference that drug melting endotherm slightly shifted from its original position of 177.82 to 176.72°C. Thermogram of NOS–β-CD inclusion complex prepared by freeze drying method (1:1) shows complete disappearance of the endothermic peaks characteristic of NOS and shifts the β-CD peak at 64.57°C. Moreover, NOS–β-CD inclusion complex (1:2) shows the β-CD peak at 85.91°C.
We next attempted to define the crystalline structure of the drug in the inclusion complex using PXRD technique. The XRD pattern of NOS showed peaks that were intense and sharp indicating its crystalline structure (Fig. 5a–e). The PM of NOS with β-CD showed undefined broad, diffused peaks with low intensities. Though this signifies amorphous structure, a few sharp peaks having less intensity were observed with 1:1 complex. Finally, the inclusion complexes of NOS with β-CD prepared by freeze drying method (1:1) showed peaks of diminished intensity. Moreover, NOS–β-CD (1:2) inclusion complex exhibited few sharp peaks suggesting that the crystallinity of the drug is retained even upon complexation. This also is suggestive that 1:1 complex formation is more favorable than the 1:2 complexation.
Molecular modeling
To estimate the structures of the complexes of NOS with β-CD on the basis of information provided by various spectroscopy techniques, we considered the two feasible structures of NOS–β-CD inclusion complex that are different in directions of penetration of NOS and in number of hydrophobic groups that are incorporated. We have listed the heat of formation of both orientations derived by semiempirical methods (AM1 and PM3) in Table 2. Our results indicate that the heat of formation of O–CH2–O– in orientation was very less compared to H3CO–C6H4–OCH3 in orientation from all theoretical methods. When O–CH2–O– in complex was formed, the 3′ hydrogen of β-CD forms hydrogen bond with oxygen atom of O–CH2–O– and the hydrogen bond distances were in 2.59 Ǻ in AM1 and 2.57 Ǻ in PM3. However, in case of H3CO–C6H4–OCH3 in complex hydrogen bond distances were 3.07 Ǻ in AM1 and 2.63 Ǻ in PM3. Thus, it is clear that O–CH2–O– in complex is more favorable than H3CO–C6H4–OCH3 in complex (Fig. 3a–c). Additionally, the heat of formation (ΔH f) of the individual component is higher than inclusion complex of NOS in β-CD, so the considered complexes are favorable.
Nuclear magnetic resonance
NMR spectroscopy provides the most direct evidence for true inclusion complex formation since H-3 and H-5 hydrogens of the β-CD, which point toward the interior of the CD cavity, are remarkably shifted. There is a significant chemical shift in protons of guest molecule (NOS) when encapsulated in β-CD as the host molecule, as reported in the Table 3. All the aromatic protons of the NOS are deshielded upon complexation. However, the O–CH2–O– protons of NOS upon complexation with β-CD are shielded. The O–CH2–O– protons become non-equivalent upon complexation, the singlet state of the O–CH2–O– protons changes to doublet state, indicating the splitting of the signal. This phenomenon was further studied by ROESY spectroscopy (Fig. 3d). The O–CH2–O– protons of NOS in ROESY spectra of NOS–β-CD inclusion complex represented by cross peaks indicate that both protons are showing the intra-molecular attraction with each other. Hence, the data of 1H NMR and ROESY revealed that when O–CH2–O– moiety of the NOS enters the β-CD cavity, then H-3 protons of the host might interact with oxygen atom of the O–CH2–O– moiety thus causing the chemically non-equivalent environment in the vicinity of β-CD. These results are in concord with molecular modeling of NOS–β-CD complexation (Fig. 3a–c), which showed the same orientation.
Solubility study
To express the potential of β-CD in solubilizing the guest molecule, the apparent solubility of NOS in the aqueous solution was measured, which was significantly (P < 0.05) increased by NOS complexation with β-CD, unlike the pure NOS that has more affinity for the non-aqueous phase. NOS–β-CD inclusion complex has a solubility of 49.3 mg/L in the aqueous phase, whereas drug alone showed solubility of only 4.76 mg/L in the aqueous phase. This represented a significant 10.35-fold increase in aqueous solubility of NOS.
Dissolution profile in acidic and basic medium
The dissolution profile of NOS, NOS hydrochloride, PM of NOS and β-CD and their inclusion complexes were analyzed at pH 1.2 and 7.4 (Fig. 6a–b). Dissolution profile at pH 1.2 suggested that NOS base is quickly dissolved at pH 1.2. NOS base showed 98.5% release from hard gelatin capsule within 15 min in comparison with NOS hydrochloride’s exhibited 91.8% release at the same time interval. Similar release profile was also presented by PM (NOS/β-CD) and released 96.66% of NOS in 15 min, which was not significantly (P > 0.05) different from NOS base. However, release of NOS base from inclusion complex was suppressed (53.5%). The dissolution profile of NOS and its inclusion complexes observed at pH 7.4 indicated that NOS was completely released (99.6%) after 120 min in comparison with PM of NOS and β-CD (12.1%) and NOS base (9.32%) at the same time interval.
a In vitro release of NOS base, PM of NOS/β-CD, NOS hydrochloride and NOS–β-CD inclusion complex in simulated gastric fluid at pH 1.2. Data are presented as mean ± S.D for n ≥ 3; P < 0.05 versus NOS–β-CD and NOS base, PM or NOS hydrochloride; NOS hydrochloride and NOS base. b Dissolution profile in PBS at pH 7.4. Results show that NOS–β-CD inclusion complex release high percentage of drug at pH 7.4 in comparison with NOS base and PM. Data are presented as mean ± S.D for n ≥ 3; P < 0.05 versus NOS–β-CD and NOS base or PM; P > 0.05 versus NOS base and PM
Pharmacokinetics upon oral administration of noscapine and its inclusion complex in mice
The plasma concentration–time profiles of NOS and NOS–β-CD inclusion complex were compared at single dose level (150 mg/kg) in male mice upon oral administration. Our previously published data show that 150 mg/kg of administration of NOS by oral route in mice exhibited maximum absolute bioavailability (42.22%) in comparison with 75 mg/kg (23.98%) and 300 mg/kg (26.68%) [6]. Hence, in the present investigation, 150 mg/kg equivalent of NOS and NOS–β-CD inclusion complex were administered to male mice and plasma concentration–time profile curve was plotted as shown in Fig. 7. The various pharmacokinetic parameters were computed and compiled in Table 4. NOS was easily absorbed, reaching a C max of 20.65 μg/ml at a t max of 2 h upon oral administration of 150 mg/kg, respectively in male mice. A significant (P < 0.05) increase in C max (91 μg/ml at a t max of 1 h) was noticed following administration of 150 mg/kg equivalent of NOS in β-CD indicating improved absorption of drug after encapsulation. The percent bioavailability (F) was calculated, which was found to be 83.83 and 44.69% for NOS–β-CD and NOS, respectively. The relative bioavailability (Fr) was also found to be 205.02% for NOS–β-CD inclusion complex. However, the t 1/2 value of NOS (1.33 h) was slightly shifted to lower value in NOS–β-CD inclusion complex (1.26 h).
Comparative plasma concentration–time profile of 150 mg/kg of NOS and equivalent of NOS encapsulated in NOS–β-CD inclusion complex in Swiss albino male mice upon oral administration. Results show that NOS–β-CD complex has higher C max in comparison with NOS. Data are presented as mean ± SD for n ≥ 3; P < 0.05 versus C max of NOS–β-CD inclusion complex and NOS
Stability study of the inclusion complex
Drug content analysis of NOS–β-CD inclusion complex in storage stability study indicated that there is no change in drug content after 3 months storage at specified conditions.
Antiproliferative activity of inclusion complex
To evaluate whether NOS upon complexation with β-CD retains the anticancer attributes of the parent molecule, we examined the effect of NOS–β-CD inclusion complex on the cell cycle of various human cancer cells (MCF-7, A549, CEM) using the DNA binding dye, propidium iodide, by flow cytometric techniques (data not shown). Our results suggested that NOS in the complexed state results in similar percentage of sub-G1 cells that are indicative of apoptosis, compared to NOS alone (data not shown).
Discussion
Complex determination in solution state
It is well appreciated that phase-solubility studies are widely accepted for evaluating drug solubility behavior. The determination of K c between drug and CD is based on the measurement of an index of changes in physico-chemical properties of a drug upon inclusion. Most methods for determining the K c values are based on the determination of concentration of drug by titration experiments [19]. Hence, the pharmaceutical applications of CD are to increase drug solubility and stability in aqueous phase. We thus choose this method for the evaluation of the effect of CD complexation on NOS solubility. The 1:1 drug/CD complex is the most common type of association where a single drug molecule is included in the cavity of one CD molecule, with a stability constant K1:1 for the equilibrium between the free and associated species. The phase-solubility diagram can be classified as AL type showing the formation of a water-soluble complex [14]. This relationship suggests a first order kinetics on the complex formation between NOS and β-CD. The K c value determined, was 0.454 mM−1, at pH 7.4, indicating the formation of a stable complex with low affinity [20]. Our data suggest that the increase in drug solubility observed was possibly due to the formation of a 1:1 inclusion complex.
Complex determination in solid state
The phase-solubility analysis of NOS–β-CD inclusion complex indicated the formation of 1:1 complexation. However, to explore the molecular mechanism in the inclusion mode, spectroscopic techniques were used to elucidate the structure of the inclusion complex and correlated with the molecular modeling. FTIR spectroscopy provided the information that NOS remains stable in the complex system as the lactone ring of the drug did not form any new chemical bond upon complexation. However, FTIR data indicated that either OCH3 or OCH2 moiety of NOS is involved in the formation of inclusion complex. Therefore, FTIR analysis instructed us that inclusion mode may be incorporated either H3CO–C6H4–OCH3 or O–CH2–O– moiety of NOS in β-CD hydrophobic cavity.
Phase-solubility analysis confirmed the stoichiometry (1:1) of complex in solution state. However, to confirm the stoichiometry in solid state, DSC analysis was carried out to measure the numerous changes in the physical constant of the drug upon complexation. Thermograms of NOS, β-CD and inclusion complexes were recorded, which indicated the disappearance of endothermic peak characteristic of NOS in 1:1 inclusion complex prepared by freeze drying method. Moreover, the 1:2 inclusion complex also exhibited the same effect, but this shift was insignificant. Hence, DSC analysis confirmed the formation of 1:1 NOS–β-CD inclusion complex in solid state.
Furthermore, PXRD analysis was conducted to express the crystalline structure of drug in inclusion complexes. Therefore, PXRD pattern of NOS, β-CD and inclusion complexes were recorded. NOS–β-CD inclusion complex (1:1) exhibited the peaks of diminished intensity in comparison with NOS showed the sharp peaks. This suggested that NOS is present in the high energetic amorphous state in NOS–β-CD inclusion complex (1:1), which is more wettable than crystalline state. This feature of NOS in inclusion complex would favor the enhancement of solubility in aqueous phase. Stability study analysis supported that the complexes were stable at 40°C/75% RH after 3 months storage.
Structure elucidation by molecular modeling and NMR spectroscopy
Physical characterization of solid complexes confirmed the formation of NOS–β-CD inclusion complex (1:1). However, to elucidate the structure of the inclusion complex, molecular modeling and NMR spectroscopy were used. Molecular mechanics calculations were carried out in gas phase by simultaneously emulating the NOS molecule in the hydrophobic cavity of the β-CD. It showed the lower minimum energy structure calculated by AM1 and PM3 methods and proposed that O–CH2–O– moiety fit better in to hydrophobic vicinity of the β-CD. It forms the hydrogen bond (lower hydrogen bond distance) with H-3 protons of β-CD cavity in comparison with H3CO–C6H4–OCH3 moiety exhibited high hydrogen bond distance. As the increased solubility of a drug in the presence of β-CD cannot be considered as a definite proof for the formation of inclusion complexes, we further performed NMR studies. NMR techniques have been widely used to investigate supramolecular assemblies in solution, their stoichiometries and structure of the resulting complexes, especially the orientation of the guest molecule in the CD cavity [21]. It is well known that the chemical shift (δ) of a given nucleus depends on its shielding constant and in turn is sensitive to medium effects. Therefore, changes in δ (ppm) values of the host and guest nuclei can provide a measure of the degree of complex formation since significant changes in the microenvironment are known to occur between free and bound states [22]. As the chemical environment of some protons changes upon complexation, there is a consequent variation in the chemical shifts (δ ppm) of 1H NMR resonance (shielding or deshielding effects). Hence, the molecular structure of the inclusion complex of NOS in β-CD (1:1) was elucidated with 1H NMR and ROESY spectroscopy. It was observed that O–CH2–O– protons of the NOS turned to non-equivalent state after inclusion in the β-CD cavity, whereas these protons were equivalent in the chemical structure of NOS. Hence, we propose that the inclusion mode involves O–CH2–O– in orientation in agreement to AM1 optimized structure. Moreover, deshielding effect on aromatic protons upon complexation suggested that NOS is deeply penetrated in the host.
Performance of the inclusion complex in dissolution profile and pharmacokinetics
The dissolution study of NOS–β-CD inclusion complex was done in order to explain the enhanced solubility of NOS in aqueous phase. Since, NOS is an alkaloid base and it is well-known fact that weakly basic drugs are soluble in acidic pH and thus protonated, caused ionization. Hence, the dissolution profile of NOS, NOS hydrochloride and inclusion complexes were investigated in both acidic and basic pH. The suppression of solubility of NOS hydrochloride at pH 1.2 might be attributed to common ion effect. However, the release of NOS from inclusion complex was also suppressed, indicating the stability of inclusion complex at acidic pH. In contrast, weakly basic drugs remain undissociated at neutral/basic pH and are absorbed by passive diffusion through lipoidal barrier. Bases in the pKa ~5–11 are greatly affected by changes in pH and hence their absorption is pH dependent [23]. NOS requires basic environment to remain in unionized form and also for its absorption. The pKa of NOS is reported to be 7.8 (opioids generally have the pKa ~6.5–8.7) [24]. Hence, NOS, a weak base remains undissociated at neutral/basic pH and incorporation of NOS in β-CD increased the solubility at basic pH. The dissolution profile of NOS and NOS–β-CD inclusion complex indicated that NOS quickly released from the inclusion complex in comparison with NOS base and PM of NOS and β-CD, which ensures that NOS will be readily absorbed from the basic environment of the GIT. Moreover, the inclusion complex gave significantly (P < 0.05) shorter t max and higher AUC and C max of NOS–β-CD than did NOS. Pharmacokinetic analysis revealed the 1.87-fold enhancement in absolute bioavailability indicating that the enhanced oral bioavailability of NOS in the inclusion complex was contributed by the marked increase in the absorption rate of NOS due to the increased dissolution rate of NOS from the inclusion complex in mice [25–27]. But no correlation was found in solubility and bioavailability. However, t 1/2 value of NOS was not significantly (P > 0.05) changed in NOS–β-CD inclusion complex and suggested that inclusion complex would be useful to deliver NOS in a pattern that allows fast absorption in the initial phase, leading to better absorption. Our results thus indicate that best oral bioavailability was observed at 150 mg/kg and this bioavailability was further improved by fabrication of inclusion complex of NOS in β-CD. Since, NOS is already in phase I/II clinical trials for anticancer therapy, the current study reveals that β-CD may be effectively utilized for the inclusion of NOS to design anticancer formulations.
References
Landen JW, Lang R, McMohan SJ, Rusan NM, Yvon AM, Adams AW, Sorcinelli MD, Campbell R, Bonaccorsi P, Ansel JC, Archer DR, Wadsworth P, Armstrong CA, Joshi HC (2002) Noscapine alters microtubule dynamics in living cells and inhibits the progression of melanoma. Clin Cancer Res 62:4109–4114
Zhou J, Gupta K, Aggarwal S, Aneja R, Chandra R, Panda D, Joshi HC (2003) Brominated derivatives of noscapine are potent microtubule-interfering agents that perturb mitosis and inhibit cell proliferation. Mol Pharmacol 63:799–807
Aneja R, Vangapandu SN, Lopus M, Chandra R, Panda D, Joshi HC (2006) Development of a novel nitro-derivative of noscapine for the potential treatment of drug resistant ovarian cancer and T-cell lymphoma. Mol Pharmacol 69:1801–1809
Aneja R, Zhou J, Zhou B, Chandra R, Joshi HC (2006) Treatment of hormone-refractory breast cancer: apoptosis and regression of human tumors implanted in mice. Mol Cancer Ther 5:2366–2377
Landen JW, Hau V, Wang M, Davis T, Ciliax B, Wainer BH, Van Meir EG, Glass JD, Joshi HC, Archer DR (2004) Noscapine crosses the blood–brain barrier and inhibits glioblastoma growth. Clin Cancer Res 10:5187–5201
Aneja R, Dhiman N, Idnani J, Awasthi A, Arora SK, Chandra R, Joshi HC (2007) Preclinical pharmacokinetics and bioavailability of noscapine, a tubulin-binding anticancer agent. Cancer Chemother Pharmacol 60:831–839
Vyas SP, Khar RK (2002) Controlled drug delivery: concepts and advances. Vallabh Prakashan, New Delhi
Pascall B, Maud G, Georges D, Adelin A, Valery B, Bruno P, Didier C, Géraldine P, Luc D, Brigitte E (2005) The effect of cyclodextrins on the aqueous solubility of a new MMP inhibitor: phase solubility, 1H-NMR spectroscopy and molecular modeling studies, preparation and stability study of nebulizable solutions. J Pharm Pharm Sci 8:164–175
Szejtli J, Szente L (2005) Elimination of bitter, disgusting taste of drugs and foods by cyclodextrins. Eur J Pharm Biopharm 61:115–125
Martin Del Valle EM (2004) Cyclodextrin and their uses: a review. Process Biochem 39:1033–1046
deAraujo MVG, Vieira EKB, Lazaro GS, deSouza.Conegero L, Ferreira OP, Almeida LE, Barreto LS, Dacosta NB Jr, Gimenez IF (2007) Inclusion complexes of pyrimethamine in 2-hydroxypropyl-β-cyclodextrin: characterization, phase solubility and molecular modeling. Bioorg Med Chem 15:5752–5759
Prabagar B, Yoo BK, Woo JS, Kim JA, Rhee JD, Piao MG, Choi HG, Yong CS (2007) Enhanced bioavailability of poorly water-soluble clotrimazole by inclusion with β-cyclodextrin. Arch Pharm Res 30:249–254
Sethi PD (1985) Analysis of drug in pharmaceutical formulations. New Age Publishing Company, New Delhi
Higuchi T, Connors AK (1965) Advances in analytical chemistry and instrumentation. Adv Anal Chem Instrum 4:117–212
Liu Y, Chen GS, Chen Y, Ding F, Chen J (2005) Cyclodextrin as carriers for cinchona alkaloids: a pH-responsive selective binding system. Org Biomol Chem 3:2519–2523
Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP (1985) Development and use of quantum mechanical molecular models. 76. AM1: a new general purpose quantum mechanical molecular model. J Am Chem Soc 107:3902–3909
Stewart JJP (1989) Optimization of parameters for semiempirical methods 1. Method. J Comput Chem 10:209–220
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven (Jr) T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian, Inc., Wallingford CT, 2004
Loftsson T, Brewster ME (1996) Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci 85:1017–1025
Loukas YL, Vraka V, Gregoriadis G (1998) Drugs, in cyclodextrins in liposomes: a novel approach to the chemical stability of drugs sensitive to hydrolysis. Int J Pharm 162:137–142
Djedaini F, Perly B (1991) Nuclear magnetic resonance of the stoichiometries in β-cyclodextrin: steroid inclusion complexes. J Pharm Sci 80:1157–1161
Wilson LD, Verral RE (1998) F and 1H NMR investigation of cyclodextrin/fluorocarbon alkyl carboxylate surfactant inclusion complexes. Langmuir 14:4710–4717
Brahamnkar DM, Jaiswal SB (1995) Biopharmaceutics and pharmacokinetic—a treatise. Vallabh Prakashan, New Delhi
Olsson B, Bolme P, Dahlstrom B, Marcus C (1986) Excretion of noscapine in human breast milk. Eur J Clin Pharmacol 30:213–215
Becket G, Schep LJ, Tan MY (1999) Improvement of the in vitro dissolution of praziquantel by complexation with α-, β-, and γ-cyclodextrin. Int J Pharm 179:65–71
Gandhi RB, Karara AH (1988) Characterization, dissolution and diffusion properties of tolbutamide-β-cyclodextrin complex system. Drug Dev Ind Pharm 14:657–682
Wong JW, Yuen KH (2001) Improved oral bioavailability of artemisinin through inclusion complexation with β-and γ-cyclodextrin. Int J Pharm 227:177–185
Acknowledgments
Authors thank Dr. Bhupinder Singh Bhoop, University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh, India, for gift sample of betacyclodextrin and Dr. Devender Kumar, Department of Chemistry, Guru Jambeshwar University of Science and Technology, Hisar, India, for their kind help in analyzing the NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madan, J., Dhiman, N., Parmar, V.K. et al. Inclusion complexes of noscapine in β-cyclodextrin offer better solubility and improved pharmacokinetics. Cancer Chemother Pharmacol 65, 537–548 (2010). https://doi.org/10.1007/s00280-009-1060-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1060-3