Abstract
Purpose
Since anticancer agents that interfere with microtubule function are in widespread use and have a broad spectrum of activity against both hematological malignancies and solid tumors, there is an urgent need to develop novel tubulin inhibitors with broader activities and avoiding drug resistance.
Methods and results
In this study, we describe the characterization of select lead compounds from a novel class of indazole-based tubulin inhibitors. Three lead compounds, TH-337, TH-482 and TH-494, exhibit potent antiproliferative activity against cell lines derived from human pancreatic carcinoma, human breast adenocarcinoma and human colorectal adenocarcinoma cells. The three compounds were also tested for cytotoxicity against a panel of clinically relevant drug resistant cancer cell lines that either overexpress the drug resistance pumps MDR-1, MRP-1 and BCRP-1 or have altered Topoisomerase II activity. TH-482 and -494 retained cytotoxic activities against all of the resistant cell lines tested; however, TH-337 exhibited decreased cytotoxicity in the cell line overexpressing BCRP-1, indicating that TH-337 is a substrate of that pump. We show that TH-482’s antiproliferative activity is due to cell cycle arrest at the G2/M phase. We demonstrate that TH-482 binds specifically to the colchicine site of tubulin and that it inhibits tubulin polymerization in vitro in a concentration-dependent manner. The in vitro anti-vascular activities of TH-482 were assessed using the HUVEC-C cell line. TH-482 inhibits in vitro neovessel formation and disrupts pre-established vessels using HUVEC-C cells. TH-482 also increases permeability of vascular endothelial cells in a concentration- and time-dependent manner.
Conclusions
TH-482 demonstrates potent in vitro efficacy as a novel tubulin-targeted anti-proliferative and anti-vascular agent and notably is more potent in antiproliferative assays than the benchmark compound combretastatin A-4. These results identify TH-482 as a potent tubulin inhibitor, and support the investigation of its in vivo efficacy and pharmacokinetic properties as the prototype of a new class of anti-tubulin agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microtubules are highly dynamic cytoskeletal elements and play a critical role in many processes in eukaryotic cells, including mitosis, cell mobility, cell shape, intracellular organelle transport and cell–cell interactions. Microtubules are composed of two tubulin subunits, α tubulin and β tubulin. Heterodimers of α and β tubulin assemble to form a short microtubule nucleus. Nucleation is followed by rapid elongation of the microtubule at both ends by addition of tubulin heterodimers.
Essential roles of microtubules in the process of mitosis make them an attractive target for anticancer drugs [1, 2]. Tubulin targeting agents are broadly classified into two classes according to their effect on tubulin-microtubule equilibrium: microtubule stabilizers, such as taxol and epothilone; and microtubule de-stabilizers, including vinca alkaloids and combretastain A-4 (CA-4) [2]. In addition to binding to tubulin, several microtubule targeting compounds have been found to alter dynamic stability of microtubules by binding to the microtubule associated proteins (e.g. estramustine) [3], and motor proteins (e.g. monastrol) [4].
Besides the ability to inhibit tumor cell proliferation, microtubule-targeting drugs have also been shown to have activity against the vasculature in tumors [5]. Tumor vasculature differs from that normal tissues. Tumor vasculature is organized in a chaotic fashion and does not follow the hierarchical branching pattern of normal vasculature. Tumor blood vessels have sluggish blood flow and lack the equilibrium between vascular growth and cellular demands, resulting in avascular and hypoxic regions of solid tumors [6]. In addition, the structure of the tumor blood vessel wall is also abnormal, which is evident by uneven vessel diameter and defects in their endothelial lining, as well as the presence of endothelial cells undergoing apoptosis [7]. In contrast, normal vasculature is organized with vessels close enough to each other to ensure adequate nutrient and oxygen supply to all cells [5]. Recently, several antitubulin agents (e.g. CA-4-phosphate, ZD6126, TZT-1027, AVE8062, MN-029, NPI-2358, 2ME2 and diarylmaleimides) [2, 8–11] have demonstrated the ability to disrupt tumor vasculature rapidly. The proposed mechanism of CA-4-phosphate for rapid tumor vascular shutdown is via compound-induced changes in endothelial-cell shape, plasma membrane blebbing, and an increase in permeability of cell monolayers. These changes in endothelial cells result in increases in vascular resistance to blood flow and increased vascular permeability and in vasoconstriction, subsequently leading to vascular shutdown [5].
One of the challenges for successful clinical use of microtubule targeting agents is drug resistance. Two major mechanisms underlie the resistance of cancer cells to microtubule-targeting agents: drug efflux transporter-mediated mechanisms and microtubule-specific mechanisms. Multidrug resistance pumps actively efflux drugs from cancer cells and confer resistance to those agents. The best-known mediators of resistance are members of the ATP-binding cassette (ABC) family of membrane transport proteins [12]. Forty-eight human ABC transporters grouped into seven subfamilies have been identified and characterized [13, 14]. The major clinically relevant mechanisms of multidrug resistance in cancer cells involve ABCB-1 (P-glycoprotein, multidrug resistant protein, MDR-1), ABCC-1 (multidrug resistance associated-protein 1, MRP-1) or ABCG-2 (breast cancer resistant protein, BCRP-1) [14]. Microtubule binding agents, such as paclitaxel and vinblastine, have been reported to be substrates of ABCB-1 [15]. The activity patterns of those agents were negatively correlated with the expression pattern of ABCB 1 across the NCI 60 cell line panel [16]. Alterations in tubulin and microtubules represent another major resistance mechanism for microtubule-targeting agents; such alterations include altered microtubule dynamics [17], tubulin mutations [18], changes in microtubule-regulatory proteins [19] and expression of tubulin isoforms [20].
We have previously reported the synthesis of a novel class of tubulin inhibitors [21]. In this study, we report that TH-482, the prototypical member of this novel class of antitubulin compounds, demonstrates cytotoxic activity against multiple human cancer cell lines. TH-482 also exhibits cytotoxicity in cells overexpressing the drug resistant pumps MDR-1, MRP-1 and BCRP-1 and in cells with altered Topoisomerase II activity. TH-482 binds to the colchicine binding site of tubulin, inhibits tubulin polymerization and arrests cells at the G2/M phase of the cell cycle. TH-482 inhibits in vitro neovessel formation, disrupts pre-established vessel formation and increases vascular endothelial permeability in vitro in a concentration- and time-dependent manner.
Materials and methods
Cell lines
The following cells lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA): H460 (Human non-small cell lung carcinoma), MIA PaCa-2 (human pancreatic carcinoma), MCF-7 (human breast adenocarcinoma), HT29 (human colorectal adenocarcinoma), HUVEC-C (human umbilical vein vascular endothelium), MESSA (human uterine sarcoma) and MESSA/DX5 (the drug resistant derivative), NCI-H69 (human small cell lung carcinoma) and H69AR (the drug resistant derivative), HL60 (human acute promyelocytic leukemia) and HL60/MX2 (the drug resistant derivative). Cells were maintained in the medium specified by the ATCC at 37°C in a humidified incubator containing 5% CO2. BCRP overexpressing cells (HEK293) were a gift from Dr. Rob Robey at the NIH and were cultured under the conditions described by Robey et al. [22].
Reagents and antibodies
[3H]-colchicine was purchased from GE Healthcare (Piscataway, NJ). AlamarBlue and goat anti-mouse FITC were purchased from Invitrogen (Carlsbad, CA). Tubulin polymerization kit, purified bovine brain tubulin, MCF-7 tubulin, Hela tubulin and F-actin visualization biochem kit were purchased from Cytoskeleton Inc. (Denver, CO). Matrigel and fibronectin-coated plates were purchased from BD Transduction Laboratories (San Diego, CA). FITC-dextran, β tubulin antibody, calcein AM and Hoechst 33258 were purchased from Sigma Aldrich (St Louis, MO). FGF-2 was purchased from R&D system (Minneapolis, MN). DE81 filters and tissue culture coverslips were purchased from VWR International (West Chester, PA). TUNEL assay kit and cell cycle reagents were purchased from Guava (Hayward, CA). G418 was purchased from the ATCC (Manassas, VA).
In vitro proliferation assay
In vitro proliferation was conducted as described by Meng et al. [23]. In brief, exponentially growing cells were seeded at a density ranging from 4 × 103 to 6 × 103 cells per well in a 96 well plate and incubated at 37°C in 5% CO2, 95% air and 100% relative humidity for 24 h prior to addition of test compounds. The cell population for each cell line at the time of drug addition was measured using the AlamarBlue assay (T 0). Compounds were solubilized in 100% DMSO at 200 times the desired final test concentration. At the time of drug addition, compounds were further diluted to four times the desired final concentration with complete medium. Aliquots of 50 μl of compounds at specified concentrations were added to microtiter wells already containing 150 μl of medium, resulting in the final drug concentration reported. After drug addition, the plates were incubated for an additional 72 h at 37°C, 5% CO2, 95% air, and 100% relative humidity. At the end of this incubation, the viable cells were quantified using AlamarBlue. Drug concentration resulting in growth inhibition of 50% (IC50) was calculated using Prism software (Irvine, CA) with the results from three independent experiments.
Immunofluorescence microscopy
HUVEC-C cells were cultured on gelatin-coated coverslips. Following overnight incubation, cells were treated with vehicle or test compounds for 60 min. After fixation with 3.7% paraformaldehyde and permeabilization with 1% Triton X-100 (Kit obtained from Cytoskeleton, Inc.), the cells were incubated with mouse β tubulin monoclonal antibody (Sigma) or rhodamine phalloidin (Cytoskeleton, Inc.) for 1 h. For tubulin microtubule visualization, the cells were subsequently incubated with goat anti-mouse-FITC for 1 h in the dark. To visualize the nuclei, the cells were washed and treated with Hoechst 33258 dye before mounting the coverslip onto a microscope slide. The cells were imaged using a 40× objective and fluorescent microscope (Nikon).
In vitro tubulin polymerization assay
In vitro tubulin polymerization assays were conducted as described by the kit manufacturer (Cytoskeleton, Inc.) based on the DAPI method of Bonne et al. [24]. In brief, TH-482 was incubated with purified bovine tubulin and buffer containing 10% glycerol and 1 mM GTP at 37°C, and the effect of TH-482 on tubulin polymerization was monitored kinetically using a fluorescent plate reader (Tecan).
Tubulin binding
Test compounds at different concentrations were incubated with purified tubulin derived from bovine brain, MCF-7 cells or Hela cells for 10 min at 37°C. [3H] colchicine was then added to the samples, which were then incubated for an additional 30 min at 37°C. To separate the bound from unbound [3H] colchicine, the samples were spotted onto DE81 filter paper, and washed three times to remove unbound [3H] colchicicne. The bound colchicine on the filter was quantified using a scintillation counter (Wallac Microbeta). [3H] colchicine was added at non-depleting concentrations to the assay (∼5% bound).
Binding affinity by isothermal titration calorimetry (ITC)
ITC was performed on a VP-ITC calorimeter at MicroCal (Northampton, MA). Tubulin was at 8 μM in PEM buffer (80 mM Na-PIPES pH 6.9, 1 mM MgCl2 and 1 mM EGTA) containing 0.05 mM GDP and 0.05 mM GTP and 1% DMSO. TH-482 and CA-4 were at 100 μM in PEM buffer containing 0.1 mM GDP and 1% DMSO. Compound solutions were titrated into 1 ml of the tubulin solution in series of 20 injections of 10 μl each at 3 min intervals at 25°C. Thermodynamic binding parameters were determined after subtracting control injection heats and concentration normalization. Data were fit to a single binding site model with Origin software (MicroCal).
Cell cycle analysis
H460 cells were seeded at a density of 1.5 × 106 cells per well in six well plates. After 24 h incubation for attachment, TH-482 at different concentrations was added and incubation for another 24 h. Cells were trypsinized, centrifuged, and fixed in 75% ethanol for 24 h. Cell cycle distribution was determined using Cell Cycle reagent (Guava Technologies) and flow cytometry (Guava EasyCyte).
Neovessel formation on a Matrigel matrix
Matrigel matrix was kept at 4°C for 16 h. Five hundred microliter of Matrigel were added to each well of a 24-well plate. After gelling at 37°C for 30 min, gels were overlaid with 500 μl of medium containing 3 × 104 HUVEC-C cells. Five nanogram per milliliter of FGF-2 and different concentrations of TH-482 were added and incubated for 6 h. The cells were stained with Calcein AM, and capillary tube formation was assessed using an inverted fluorescent microscope (Nikon). To explore the effect of TH-482 on pre-established vasculature, HUVEC-C cells were seeded and incubated over Matrigel for 6 h to allow the capillary tubes to form. Then TH-482 was added and continuously incubated for additional 2 h, the disappearance of existing vasculature was monitored with fluorescent microscopy (Nikon).
Calcein AM cell viability assay
The viability of HUVEC-C cells following either 6 h or 2 h exposure to compounds was determined using the Calcein AM assay. HUVEC-C cells were seeded in 96 well plate and incubated at 37°C for 24 h. Cells were treated with vehicle or compounds at the indicated concentrations for either 2 h or 6 h. 1 μM of Calcein AM staining solution in Hank’s balanced salt solution (HBSS) was prepared, and added to each well. After incubation at 37°C for 30 min, the fluorescent intensity was measured at a wavelength of 530 nm.
Endothelial cell monolayer permeability assay
HUVEC-C cells were seeded at a density of 3 × 104 cells per well into 24-well insert wells and incubated for 3 days to allow a confluent cell monolayer to form. TH-482 at varying concentrations was added to the cells at the upper chamber and incubated for 15 min at 37°C. Subsequently, FITC-dextran was added to the upper chamber. The effect of TH-482 on HUVEC-C monolayer permeability was monitored using a fluorescent plate reader as measured by increased fluorescent signal in the lower chamber as a function of time.
Results
TH-337, TH-482 and TH-494 inhibit in vitro proliferation of multiple tumor cell lines
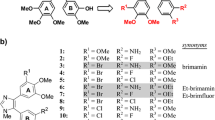
As previously described [21], TH-337, TH-482 and TH-494 (Fig. 1) inhibited proliferation of select human cancer cell lines with IC50s of low nanomolar concentration. To explore the general antiproliferative activity of these three compounds, we extended our observation to three additional human cancer cell lines: human pancreatic cancer cells (MIA PaCa-2), human breast cancer cells (MCF-7) and human colorectal cancer cells (HT29). As shown in Table 1, TH-337, TH-482 and TH-494 and CA-4, a known tubulin inhibitor, exhibited cytotoxic effects against MIA PaCa-2 and MCF-7 cell lines. TH-337 and TH-482 displayed greater potency than TH-494 and CA-4. TH-337, TH-482 and TH-494 also exhibited potent cytotoxic activity against the human colon cancer cell line HT29, which is highly resistant to CA-4. The CA-4 data are consistent with the National Cancer Institute (NCI) screening data generated from the Developmental Therapeutics Program (DTP) 60 cell lines (NSC613729 for CA-4) and published data [25].
Multiple drug resistant cell lines are sensitive to TH-482 and TH-494, but TH-337 is a substrate of ABCG2 efflux pump
To investigate whether TH-337, TH-482 and TH-494 are substrates of drug efflux pumps, the compounds were tested against a panel of drug resistant cell lines which either overexpress P-glycoprotein (ABCB-1; MDR-1), multidrug resistance-associated protein (ABCC-1; MRP-1), breast-cancer resistant protein (ABCG2; BCRP-1) or are associated with altered Topoisomerase II catalytic activity. The MESSA/DX5 cell line is highly resistant to daunorubicin, paclitaxel (Taxol) and colchicine due to overexpression of MDR-1 [26]. H69AR is highly resistant to daunorubicin due to overexpression of MRP-1 [27]. HEK293 cells stably transfected with ABCG-2 are highly resistant to mitoxantrone and topotecan [22]. HL-60/MX2 is resistant to mitoxantrone due to altered Topoisomerase II catalytic activity and reduced levels of topoisomease II α and β [28]. As shown in Table 2, TH-482 and TH-494 exhibited cytotoxic activity across all four of these clinically relevant drug resistant cell lines, which indicates that TH-482 and TH-494 are not substrates for the drug resistant pumps examined. BCRP-1 overexpressing cells were 30-fold more resistant to TH-337 than cells transfected with vector only, suggesting that TH-337 is a substrate of the BCRP efflux pump. The resistant properties of these cell lines were confirmed using the corresponding positive control compounds (Table 2). Daunorubicin, a known substrate of P-glycoprotein (MDR-1) and multiple resistant protein (MRP-1), demonstrated less activity against the drug resistant derivative cells relative to the sensitive parental cells. Mitoxantrone and topotecan, known substrates for BCRP-1 efflux pump, exhibited 40-fold less activity against cells overexpressing BCRP relative to cells transfected with empty vector. The topoisomerase II-targeting agent mitoxantrone showed less activity against drug resistant HL-60/MX2 relative to the parental sensitive cell line HL-60 (Table 2).
Concentration-dependent microtubule depolymerization by TH-482
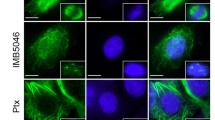
Proliferating HUVEC-C cells were treated with varying concentrations of TH-482 for 24 h, and then, microtubules and F-actin filaments were visualized by fluorescence microscopy. As shown in Fig. 2a and b, TH-482 at concentrations as low as 3 nM was able to disrupt polymerization and actin organization in proliferating HUVEC-C cells, as demonstrated by the disruption of the microtubule network and F-actin filamentous structures. A higher concentration (10 nM) of the known tubulin inhibitor CA-4 was required to disrupt the microtubule network and actin filaments (Fig. 2c and d).
Concentration-dependent disruption of microtubules and actin filaments. Proliferating HUVEC-C cells were treated with various concentrations of either TH-482 (a and b) or CA-4 (c and d) for 24 h. Microtubules (a and c) and actin filaments (b and d) were visualized using fluorescence microscopy. Magnification: ×40. Scale bar, 10 μm. The data are the representative of three independent experiments
We also used a cell-free tubulin polymerization assay to examine the effects of TH-482 on microtubule formation; in this assay microtubule formation in vitro is monitored by the increase in fluorescent intensity of the reaction mixture. As shown in Fig. 3a, in the absence of TH-482, fluorescent intensity increases as the tubulin polymerization reaction proceeds. In contrast, addition of TH-482 inhibits tubulin polymerization in a concentration-dependent manner, with an IC50 value of approximately 6 μM, and demonstrates complete inhibition at 20 μM. CA-4 displays a similar inhibition of in vitro tubulin polymerization, also in the low micromolar range (Fig. 3b).
Inhibition of in vitro tubulin polymerization and induction of mitotic arrest by TH-482 (a and c) and CA-4 (a and d). Purified tubulin and tubulin inhibitors were preincubated together for 10 min and then tubulin polymerization was monitored kinetically using the DAPI fluorophore as a probe and a fluorescence plate reader (a and b). Compound effects on cell cycle distribution were characterized using cell cycle reagent and flow cytometry (c and d) after H460 cells treated with compound for 24 h. The data are the representative of three independent experiments
TH-482 arrests cell cycle at G2/M phase
The ability of TH-482 to modulate cell cycle progression was evaluated. H460 cells were exposed continuously to TH-482 for 24 h and the effects on cell cycle distribution were analyzed using flow cytometry. Treatment with varying concentrations of TH-482 (Fig. 3c) and CA-4 (Fig. 3d) also led to the accumulation of cells at the G2/M. Under these conditions of mitotic arrest, cells were rounded in appearance and loosely adherent, indicating a disruption of the cytoskeleton. In contrast, cells treated with lower concentrations of TH-482 (1.2 nM or less) or with vehicle-only remained strongly adherent and normally distributed throughout the cell cycle. As shown in Table 3, the concentration required for TH-482-induced cell cycle arrest is lower than that required for CA-4. Nocodazole, used as a positive control, arrested 80% of the cells at G2/M phase (data not shown).
TH-482 specifically binds to the colchicine site of tubulin
To identify the TH-482 binding site on tubulin, the ability of TH-482 to compete with [3H] colchicine binding to tubulin was examined. As shown in Fig. 4a, TH-482 inhibited colchicine binding to bovine brain tubulin in a concentration-dependent manner (IC50 of 47 nM). In the same experiment, CA-4 inhibited colchicine binding with lower potency (IC50 of 181 nM; Fig. 4a). Similarly, TH-482 was also able to compete with [3H] colchicine binding to purified tubulin derived from Hela cells and MCF-7 cells. As shown in Fig. 4b, TH-482 inhibited colchicine binding to Hela tubulin and MCF-7 tubulin with IC50 values of 32 and 56 nM, respectively.
Inhibition of colchicine binding by TH-482 and CA-4 (a and b) and tubulin binding properties of TH-482 (c) and CA-4 (d) as measured by ITC. Purified bovine brain tubulin (a) was preincubated with TH-482 (IC50 of 47 nM) or CA-4 (IC50 of 181 nM) for 10 min at 37°C. Purified tubulin from either Hela cells (IC50 of 32 nM) or MCF-7 cells (IC50 of 56 nM), was preincubated with TH-482 (b). [3H] colchicine was then added to the reaction. The samples were spotted on DE81 filters, and bound and unbound [3H] colchicine separated by washing three times. The bound [3H] colchicine was quantified using a scintillation counter. The binding properties of TH-482 (c) and CA-4 (d) to tubulin were studied by ITC. Both purified tubulin and compounds were diluted in PEM buffer. ITC was performed in a series of 20 injections of 10 μl each at 3 min intervals at 25°C. The data are the representative of two independent experiments
Binding affinity of TH-482
The binding affinity of TH-482 to tubulin was determined by isothermal titration calorimetry (ITC) [29]. As shown in Fig. 4c, a binding constant (K) of 4.3 × 106 M−1 (K d = 0.23 μM) was obtained. The binding enthalpy (ΔH) was estimated to be −3.2 kcal/mole. The binding entropy (ΔS) was calculated to be 19.6 cal mol−1K−1. Thus the binding of TH-482 is driven by both favorable enthalpy and entropy changes. Compared to TH-482, the binding affinity of CA-4 to tubulin was nearly identical (K d = 0.29 μM) (Fig. 4d). For CA-4, the binding enthalpy (ΔH) was −7.3 kcal/mole and the binding entropy (ΔS) was 5.5 cal mole−1 K−1. The larger positive entropy change for TH-482 binding suggests that positive entropy changes play a greater role in TH-482 binding.
TH-482 inhibits neovessel formation and disrupts pre-established vessels in vitro
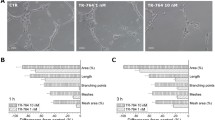
In vitro vascular tube formation is reflected by the migration and alignment of vascular endothelial cells in a capillary-like structure and involves multiple steps, including morphological change, cell migration and cytoskeleton reorganization. As shown in Fig. 5A, when HUVEC-C cells were plated on Matrigel in the absence of TH-482, they migrated and aligned with one another and formed capillary tube-like structures. In contrast, addition of TH-482 at 2.7 nM or higher concentrations resulted in a complete inhibition of tube formation after 6 h treatment (Fig. 5a). Cells remained isolated, and few aggregated cells were observed. A similar effect with CA-4 was also observed, although a higher concentration was required to achieve the same effect (Fig. 5b).
Inhibition of neovessel formation and pre-established vasculature by TH-482 (a and c) and CA-4 (b and d). Matrigel was layered into the well, and HUVEC-C cells were seeded on top of it. Tubulin inhibitors were added at the indicated concentrations, and incubated with HUVEC-C cells for 6 h. To study the effect of TH-482 on pre-established vasculature, HUVEC-C cells were seeded on matrigel, and cultured to form vessel-like capillary structures (6 h). Cells were then treated with TH-482 and CA-4 for 2 h. The effect of tubulin inhibitors was visualized using Calcein AM as stain and fluorescence microscopy. The data are the representative of three independent experiments
To explore the effect of TH-482 and CA-4 on established vessels, we formed vessels in vitro and treated them with the tubulin inhibitors. As shown in Fig. 5c, TH-482 disrupted the pre-established capillary-like structures in a concentration-dependent manner after 2 h treatment. A similar effect was observed in cells treated with CA-4 (Fig. 5d). Under the similar experimental conditions, no cytotoxic effects of TH-482 and CA-4 on HUVEC-C cells were observed using a Calcein AM viability assay (data not shown).
TH-482 increases permeability of the vascular endothelial monolayer
Changes in endothelial cell monolayer permeability also provide an in vitro model of tumor vascular disruption. The compound-induced increase in permeability of a confluent HUVEC-C monolayer was monitored with FITC-dextran. As shown in Fig. 6a, vehicle-alone treatment did not disrupt the integrity of the HUVEC-C monolayer, which was evident by the low fluorescent signal from the lower well throughout the experimental time of 120 min. In contrast, significant increases in endothelial cell permeability were observed when the confluent monolayers were treated with 10 and 100 nM TH-482 (Fig. 6a). TH-482 increased permeability of the vascular endothelial monolayer in a concentration- and time-dependent manner. CA-4 displayed a similar profile (Fig. 6b), although with lower potency.
Concentration- and time-dependent induction of HUVEC-C monolayer permeability by TH-482 (a) and CA-4 (b). HUVEC-C cells were seeded into fibronectin-coated insert well and incubated for 3 days to achieve a confluent cell monolayer. TH-482 and CA-4 at the indicated concentrations were added and incubated for 15 min at 37°C. Subsequently, FITC-dextran was added to the upper chamber. The fluorescence within the lower chambers was monitored kinetically using fluorescence plate reader. The data are the representative of three independent experiments
Discussion
In this study, we show that TH-337, TH-482 and TH-494 are potent cytotoxic agents against the human cancer cell lines MIA PaCa-2, MCF-7 and HT29. TH-482 and TH-494 also exhibit cytotoxic activity in cell lines overexpressing the drug efflux pumps MDR-1, MRP-1 and BCRP-1 and in cells with altered topoisomerase activity. In contrast, TH-337 shows decreased cytotoxicity in a cell line overexpressing BCRP-1, suggesting that TH-337 is a substrate of BCRP-1 efflux pump. TH-482 is the most potent antiproliferative compound in all eleven cell lines examined to date. TH-482 binds to the colchicine site of tubulin, disrupts tubulin polymerization in vitro and arrests cell cycle at the G2/M phase. In addition, TH-482 inhibits in vitro formation of a capillary-like structure formed by an endothelial cell line, disrupts pre-existing vessels, and increases permeability of vascular endothelial cells.
TH-482 exhibited potent antiproliferative activity against HT-29 cells, which are highly resistant to CA-4. One hypothesis for the CA-4 resistance in HT29 is significant glucuronidation of CA-4 by glucuronosyl transferase. It has been reported that high glucuronosyl transferase activity is observed in colorectal cancer cells and contributes to resistance to drugs such as mycophenolic acid [30]. To address whether TH-482 is a substrate for drug efflux pumps, we selected a panel of resistant cell lines with differential resistant mechanisms, including cell lines overexpressing MDR-1, MRP-1 and BCRP-1 and cell lines with altered Topoisomerase II activity. It has been reported that vinblastine, paclitaxel and their respective analogs are substrates of MDR-1 [13]. Our data showed that there were no differential cytotoxic activities observed between sensitive and resistant cell lines overexpressing MDR-1, MRP-1 or cell lines with altered Topoisomerase II for TH-337, TH-482 and TH-494, suggesting that they are not substrates for those drug resistance mechanisms. However, TH-337 is a substrate of the BCRP pump, as apparent by its exhibiting 30 fold less activity against cells overexpressing BCRP. In contrast, TH-484 and TH-494 maintain their efficacy in the BCRP overexpressing cell line.
Inhibition of cellular activities, including cell proliferation, cell cycle arrest and disruption of microtubule networks and actin filaments require only low nanomolar concentrations of TH-482. In contrast, the concentration of TH-482 required for inhibition of in vitro microtubule polymerization is in the micromolar range. Similar observations have been reported for other tubulin inhibitors, including epithilones [31], paclitaxel [32], CA-4 sulfonate analogs [33], 2-(3′,4′,5′-trimethoxybenzoyl)-3-amino 5-aryl thiophenes [34, 35] and T138067 [36]. The basis for this difference has been attributed to intracellular retention of the compounds, leading to higher intracellular concentrations [32]. It has also been proposed that the cellular response depends more on microtubule dynamics effects than solely on the inhibition of tubulin polymerization [2]. In addition, the difference between long-term continuous treatment in the cell-based assay and instantaneous measurements in the biochemical tubulin polymerization assay can theoretically explain such potency differences [37]. We hypothesize that similar mechanisms underlies the observed TH-482 potency differences between cell-based and cell-free assay formats.
Abnormal vasculature is a hallmark of solid tumors [5]. Tumor vascular networks are disorganized, and the vessel walls are poorly developed and highly permeable, with irregular and abnormal basement membranes. Endothelial cells in tumor blood vessels do not express common endothelial markers and undergo apoptosis [38]. In addition, tumor blood vessels are often far from each other and have sluggish blood flow. Tubulin-binding agents have selectively damaging effects on tumor vasculature and can cause hemorrhage and tumor necrosis [39]. For example, vinblastine, vincristine and colchicine administered intraperitoneally to B6D2F1 mice with advanced subcutaneous colon 38 tumor substantially reduced tumor blood flow and tumour growth [40]. A similar observation was reported with the murine carcinoma CaNT model [41]. A moderate dose of CA-4 caused a significant reduction in tumor blood flow within 5 minutes of drug administration, and almost complete vascular shutdown was achieved within 20 minutes [42]. The effects of CA-4-P resembled an acute inflammatory reaction, resulting in a visible loss of a large proportion of the smallest blood vessels, and hematocrit and tumor vascular permeability were increased [42]. We explored the antivascular effect of TH-482 using the HUVEC-C model system. The HUVEC-C cell line shares many characteristics with primary endothelial cells, including in vitro capillary formation and proliferative and differentiative response to growth factors [43]. The formation of capillary tubes in vitro involves cell–cell interactions, the reorganization of the cytoskeleton, cell migration and alignment [44]. In this study, we show that TH-482 blocks formation of capillary tube networks in a concentration-dependent manner. In addition, TH-482 is also able to disrupt a pre-established in vitro vasculature in a concentration-dependent manner.
TH-482 not only inhibits vascular endothelial cell tube formation but also increases endothelial cell monolayer permeability. The vascular permeability effects in this study mimic some the effects of tumor vascular collapse in vivo [9]. The endothelial cell lining of the internal vasculature defines a semi-permeable barrier between the blood and the interstitial spaces of the body. Disruption of the barrier integrity is manifested as microvascular hyperpermeability. When confluent monolayer HUVEC-C cells were treated with TH-482, a concentration- and time-dependent increase in permeability was observed. The ability of TH-482 to inhibit vessel formation and induce vascular permeability is consistent with anti-vascular cancer agent utility and warrants further testing of this compound in preclinical in vivo cancer models.
Abbreviations
- MDR-1:
-
Multi-drug resistant-1
- MRP-1:
-
Multidrug resistance-associated protein-1
- BCRP:
-
Breast cancer resistant protein
- CA-4:
-
Combretastatin A-4
- ITC:
-
Isothermal titration calorimetry
- PEM:
-
Pipes, EGTA and MgCl2 (general tubulin buffer)
References
Hadfield JA, Ducki S, Hirst N et al (2003) Tubulin and microtubules as targets for anticancer drugs. Prog Cell Cycle Res 5:309–325
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Yoshida D, Hoshino S, Shimura T et al (2000) Drug-induced apoptosis by anti-microtubule agent, estramustine phosphate on human malignant glioma cell line, U87MG; in vitro study. J Neurooncol 47:133–140
Mayer TU, Kapoor TM, Haggarty SJ et al (1999) Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286:971–974
Tozer GM, Kanthou C, Baguley BC (2005) Disrupting tumour blood vessels. Nat Rev Cancer 5:423–435
Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9:685–693
Chang YS, di Tomaso E, McDonald DM et al (2000) Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA 97:14608–14613
Lippert JW (2007) Vascular disrupting agents. Bioorg Med Chem 15:605–615
Nicholson B, Lloyd GK, Miller BR et al (2006) NPI-2358 is a tubulin-depolymerizing agent: in-vitro evidence for activity as a tumor vascular-disrupting agent. Anticancer Drugs 17:25–31
Peifer C, Stoiber T, Unger E et al (2006) Design, synthesis, and biological evaluation of 3,4-diarylmaleimides as angiogenesis inhibitors. J Med Chem 49:1271–1281
Davis PD, Dougherty GJ, Blakey DC et al (2002) ZD6126: a novel vascular-targeting agent that causes selective destruction of tumor vasculature. Cancer Res 62:7247–7253
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2:48–58
Szakacs G, Annereau JP, Lababidi S et al (2004) Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell 6:129–137
Szakacs G, Paterson JK, Ludwig JA et al (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5:219–234
Lee JS, Paull K, Alvarez M et al (1994) Rhodamine efflux patterns predict P-glycoprotein substrates in the National Cancer Institute drug screen. Mol Pharmacol 46:627–638
Shoemaker RH (2000) Genetic and epigenetic factors in anticancer drug resistance. J Natl Cancer Inst 92:4–5
Goncalves A, Braguer D, Kamath K et al (2001) Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc Natl Acad Sci USA 98:11737–11742
Giannakakou P, Sackett DL, Kang YK et al (1997) Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem 272:17118–17125
Zhang CC, Yang JM, White E et al (1998) The role of MAP4 expression in the sensitivity to paclitaxel and resistance to vinca alkaloids in p53 mutant cells. Oncogene 16:1617–1624
Kavallaris M, Kuo DY, Burkhart CA et al (1997) Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest 100:1282–1293
Duan JX, Cai X, Meng F et al (2007) Potent antitubulin tumor cell cytotoxins based on 3-aroyl indazoles. J Med Chem 50:1001–1006
Robey RW, Honjo Y, Morisaki K et al (2003) Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer 89:1971–1978
Meng F, Nguyen XT, Cai X et al (2007) ARC-111 inhibits hypoxia-mediated hypoxia-inducible factor-1 alpha accumulation. Anticancer Drugs 18:435–445
Bonne D, Heusele C, Simon C et al (1985) 4′,6-Diamidino-2-phenylindole, a fluorescent probe for tubulin and microtubules. J Biol Chem 260:2819–2825
Cushman M, Nagarathnam D, Gopal D et al (1992) Synthesis and evaluation of analogues of (Z)-1-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)ethene as potential cytotoxic and antimitotic agents. J Med Chem 35:2293–2306
Harker WG, Sikic BI (1985) Multidrug (pleiotropic) resistance in doxorubicin-selected variants of the human sarcoma cell line MES-SA. Cancer Res 45:4091–4096
Hipfner DR, Gauldie SD, Deeley RG et al (1994) Detection of the M(r) 190,000 multidrug resistance protein, MRP, with monoclonal antibodies. Cancer Res 54:5788–5792
Harker WG, Slade DL, Drake FH et al (1991) Mitoxantrone resistance in HL-60 leukemia cells: reduced nuclear topoisomerase II catalytic activity and drug-induced DNA cleavage in association with reduced expression of the topoisomerase II beta isoform. Biochemistry 30:9953–9961
Ruben AJ, Kiso Y, Freire E (2006) Overcoming roadblocks in lead optimization: a thermodynamic perspective. Chem Biol Drug Des 67:2–4
Franklin TJ, Jacobs V, Jones G et al (1996) Glucuronidation associated with intrinsic resistance to mycophenolic acid in human colorectal carcinoma cells. Cancer Res 56:984–987
Lichtner RB, Rotgeri A, Bunte T et al (2001) Subcellular distribution of epothilones in human tumor cells. Proc Natl Acad Sci USA 98:11743–11748
Jordan MA, Wendell K, Gardiner S et al (1996) Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res 56:816–825
Gwaltney SL 2nd, Imade HM, Barr KJ et al (2001) Novel sulfonate analogues of combretastatin A-4: potent antimitotic agents. Bioorg Med Chem Lett 11:871–874
Romagnoli R, Baraldi PG, Remusat V et al (2006) Synthesis and biological evaluation of 2-(3′,4′,5′-trimethoxybenzoyl)-3-amino 5-aryl thiophenes as a new class of tubulin inhibitors. J Med Chem 49:6425–6428
Romagnoli R, Baraldi PG, Pavani MG et al (2006) Synthesis and biological evaluation of 2-amino-3-(3′,4′,5′-trimethoxybenzoyl)-5-aryl thiophenes as a new class of potent antitubulin agents. J Med Chem 49:3906–3915
Shan B, Medina JC, Santha E et al (1999) Selective, covalent modification of beta-tubulin residue Cys-239 by T138067, an antitumor agent with in vivo efficacy against multidrug-resistant tumors. Proc Natl Acad Sci USA 96:5686–5691
Tron GC, Pirali T, Sorba G et al (2006) Medicinal chemistry of combretastatin A4: present and future directions. J Med Chem 49:3033–3044
Helmlinger G, Netti PA, Lichtenbeld HC et al (1997) Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol 15:778–783
Tozer GM, Prise VE, Wilson J et al (1999) Combretastatin A-4 phosphate as a tumor vascular-targeting agent: early effects in tumors and normal tissues. Cancer Res 59: 1626–1634
Baguley BC, Holdaway KM, Thomsen LL et al (1991) Inhibition of growth of colon 38 adenocarcinoma by vinblastine and colchicine: evidence for a vascular mechanism. Eur J Cancer 27:482–487
Hill SA, Lonergan SJ, Denekamp J et al (1993) Vinca alkaloids: anti-vascular effects in a murine tumour. Eur J Cancer 29A:1320–1324
Tozer GM, Prise VE, Wilson J et al (2001) Mechanisms associated with tumor vascular shut-down induced by combretastatin A-4 phosphate: intravital microscopy and measurement of vascular permeability. Cancer Res 61:6413–6422
Gifford SM, Grummer MA, Pierre SA et al (2004) Functional characterization of HUVEC-CS: Ca2+ signaling, ERK 1/2 activation, mitogenesis and vasodilator production. J Endocrinol 182:485–499
Vincent L, Kermani P, Young LM et al (2005) Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J Clin Invest 115:2992–3006
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meng, F., Cai, X., Duan, J. et al. A novel class of tubulin inhibitors that exhibit potent antiproliferation and in vitro vessel-disrupting activity. Cancer Chemother Pharmacol 61, 953–963 (2008). https://doi.org/10.1007/s00280-007-0549-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0549-x