Abstract
Purpose
The dose limiting toxicity of oxaliplatin (l-HOP) is neurotoxicity, which is characterized by an acute neuropathy and a clinically distinct chronic neuropathy. This randomized study evaluated if prolonged l-HOP infusion over the conventional l-HOP schedule was useful in reducing acute and possibly chronic l-HOP induced neurotoxicity in colon and gastric cancer patients receiving l-HOP-based regimen as adjuvant chemotherapy.
Methods
Sixty-four patients were randomly assigned to group A (26 colon and 6 gastric cancer) and to group B (23 colon and 9 gastric cancer). Chemotherapy in both groups consisted of l-HOP 85 mg/m2 i.v. only on day 1, with leucovorin 100 mg/m2 i.v. as a 2-h infusion followed by bolus 5-fluorouracil (5-FU) 400 mg/m2/day and a 22-h infusion of 5-FU 600 mg/m2/day, repeated for two consecutive days every 2 weeks for a maximum of 12 cycles. Patients in group A received l-HOP as a continuous 6-h i.v. infusion, and patients in group B received l-HOP as the conventional 2-h i.v. infusion.
Results
The percentage of patients presenting with grade ≥2 neurotoxicity was statistically lower in group A than in group B (28.1% vs. 59.3%: P = 0.02). There was a statistically lower percentage of cycles with grade ≥2 neurotoxicity in group A (6.1%) than in group B (18.5%) (P < 0.001).
Conclusions
This study suggests that l-HOP as a continuous 6-h infusion is useful in preventing and reducing acute l-HOP induced neurotoxicity in patients with colon and gastric cancer receiving FOLFOX-4 regimen as adjuvant treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
FOLFOX-4, a bimonthly combination of oxaliplatin (l-HOP), leucovorin (LV), and bolus plus infusional 5-fluorouracil (5-FU), results in response rates and time to disease progression that are superior to those achieved with LV/5-FU in metastatic colorectal cancer [1]. In the setting of adjuvant therapy, the recent randomized MOSAIC trial demonstrated that 12 FOLFOX-4 cycles reduced the risk of recurrence in patients who had undergone curative resection for colon cancer [2]. The l-HOP/LV/5-FU regimen has shown promising activity and an acceptable safety profile also in locally advanced or metastatic gastric cancer patients, and, in the near future, l-HOP-based chemotherapy will likely be evaluated as adjuvant therapy also in this type of disease [3-5].
The dose limiting toxicity of l-HOP is neurotoxicity, which is characterized by an acute neuropathy and a clinically distinct chronic neuropathy. Acute neurotoxicity, including cold-related dysesthesia and sometimes accompaniment of muscle contractions, can occur during or within the hours following l-HOP administration [1, 6]. It is characterized by peripheral nerve hyperexcitability, and is rapidly reversible without permanent sequelae [7]. Cumulative chronic sensory neuropathy produces distal dysesthesia and paresthesia that occur between treatment cycles, and increase in intensity and duration with the cumulative dose [8].
L-HOP-induced cumulative neurotoxicity develops progressively in 10–15% of patients after a cumulative dose of 780–800 mg/m2, corresponding to ∼9 cycles of an 85 mg/m2 dose, which is the dose used in FOLFOX-4: severe neuropathy with L-HOP based regimens has been reported in the range of 17–21% in the majority of trials [1, 9].
Although l-HOP-induced acute neurotoxicity is rapidly reversible and severe neurotoxicity usually improves or completely regresses after discontinuation of treatment, neurotoxic effects frequently interfere with patients’ comfort and autonomy. As l-HOP is increasingly utilized as component of adjuvant treatment in colon cancer and other tumors, neurotoxicity remains the principle discomforting side effect.
Among the various attempts to reduce the incidence of l-HOP related neurological symptoms, prolongation of infusion has been suggested to prevent pharyngolaryngeal dysesthesia, which is particularly disturbing for patients [10]. Interesting findings were described when l-HOP was continuously infused for 6 h, with an observed lower incidence of pharyngolaryngeal dysesthesia and functional impairment than that of the conventional 2-h infusion [11].
We also observed in clinical practice that a prolonged infusion time of l-HOP was apparently useful in reducing the onset of acute neurotoxicity in some patients with metastatic colorectal and gastric cancer receiving an l-HOP-based chemotherapy. These encouraging observations, concomitantly with the emerging results of MOSAIC study, prompted our investigation of a prolonged infusion of l-HOP also in the adjuvant setting.
A randomized study was designed to evaluate if prolonged l-HOP infusion over the conventional l-HOP schedule was useful in preventing and reducing acute and possibly chronic l-HOP induced neurotoxicity in colon and gastric cancer patients receiving FOLFOX-4 regimen as adjuvant chemotherapy.
Patients and methods
Eligible patients
Patients were eligible if they had undergone complete resection of histologically proven stage II/III colon cancer and/or gastric adenocarcinoma with lymph node involvement. Gastric cancer patients with T4N1-3M0, or T1-3N3M0 disease, defined as stage IV by the American Joint Committee on Cancer (AJCC) TNM staging, were also considered suitable for adjuvant treatment and were enrolled in the study.
Other eligibility criteria included: an age between 18 and 70 years, an ECOG performance status of 0-1 and a normal neurologic examination, no prior chemotherapy, adequate hematological parameters (an absolute neutrophil count of ≥1.5 × 109/l and a platelet count of ≥100 × 109/l), creatinine and total bilirubin levels <1.25 times the upper normal limit, aspartate and ALT <3.0 times the upper normal limit, the absence of a second primary tumor other than non-melanoma skin cancer or in situ cervical carcinoma. Patients with severe cardiac dysfunction, chronic diarrhea or uncontrolled sites of infection were excluded from the study. Patients were also excluded if they had history or complaints of peripheral sensory or motor neuropathy, diabetic neuropathy, or if they received vitamin B1, B6, or B12 supplements.
Written informed consent was obtained from all patients, and the study was approved by the local Ethics and Scientific Committee.
Patient evaluation
The pre-treatment evaluation, performed within the 2 weeks preceding study entry, included a detailed history and physical and neurologic examination, a complete blood cell count with differential and platelets, whole blood chemistry, the determination of tumor markers, and computed tomography (CT) scans and/or magnetic resonance imaging (MRI) of the chest and abdomen. During treatment, a clinical assessment and a complete blood cell count with differential and platelets were performed every 2 weeks. A physical examination, routine biochemical tests, and evaluation of neurotoxicity were performed every month for 12 months after the end of chemotherapy.
The assessment of recurrence was made by means of CT scan and/or MRI, repeated every 6 months for 5 years or sooner if clinically indicated. Tumor markers were measured every 3 months for the first 2 years and every 6 months thereafter for other 3 years.
The progression-free survival (PFS) was the interval between the start of treatment and the date on which disease progression was first documented. Survival was measured from the date of the start of treatment to the date of death. Follow-up was measured from the date of the first treatment administration to the date of the last contact or death.
Treatment delivery
Patients were randomly assigned to group A or group B. Chemotherapy in both groups consisted of l-HOP 85 mg/m2 i.v. only on day 1, with LV 100 mg/m2 i.v. as a 2-h infusion followed by bolus 5-FU 400 mg/m2/day and a 22-h infusion of 5-FU 600 mg/m2/day, repeated for 2 consecutive days every 2 weeks. Patients in group A received l-HOP as a continuous 6-h intravenous infusion (from 1,000 to 1,600), and patients in group B received l-HOP as the conventional 2-h intravenous infusion. Twelve cycles of treatment were planned. Treatment had to be started within 8 weeks after surgery. In order to prevent nausea and vomiting, intravenous (i.v.) 5-hydroxytryptamine-3 antagonists plus dexamethasone 8 mg i.v. were administered before the infusion. Oral loperamide 2 mg every 2 h and oral rehydration were prescribed in the case of delayed diarrhea. No cytokine prophylactic treatment was recommended.
Neuroxicity and other treatment-related toxicities
Toxicity was assessed using the NCI common toxicity criteria, Version 2.0. For toxic effects, all of the patients who had received at least one chemotherapy cycle were considered evaluable.
Neurotoxicity was evaluated by a team of neurologists who did not know the administered chemotherapy schedule. The scale for grading neurotoxicity was a specific l-HOP neurotoxicity scale: grade 1 described dysesthesia or paresthesia that completely regressed before the next cycle of therapy, grade 2 dysesthesia or paresthesia persisting between therapy courses, and grade 3 toxicity indicated dysesthesia or paresthesia causing functional impairment [12]. During adjuvant therapy patients were asked every 2-week cycle about the eventual presence of subjective symptoms: their nature, relationship to cold, location, time course, and severity (presence of functional impairment). Additionally, a standardized neurologic examination including the testing of exteroceptive sensation at hands and feet, and testing of proprioceptive sensation was performed. After the end of chemotherapy, neurotoxicity was evaluated monthly during follow-up.
The 5-FU dose was reduced by 25% after grade ≥3 diarrhoea, stomatitis, or dermatitis occurred. The l-HOP dose was reduced by 25% if grade ¾ neutropenia occurred, and in cases of persistent (≥14 days) paresthesia or temporary (7–14 days) painful paresthesia or functional impairment. In cases of persistent (≥14 days) painful paresthesia or functional impairment, l-HOP was omitted from the regimen until recovery.
Statistical analysis
Considering that about 50% of patients suffer from grade ≥2 neurotoxicity during l-HOP administration, a sample size of at least 64 patients (32 per group) would be sufficient to evaluate a 35% reduction of grade ≥2 neurotoxic effects for a new treatment schedule, with alpha and beta errors of 0.05 and 0.1, respectively. Differences in the incidence of specific neurotoxic symptoms between the two treatment groups were evaluated using the χ2-test. Cumulative toxicity, the distribution of the PFS, and the time to death were compared using Kaplan–Meier and log-rank testing.
Results
Between September 2003 and June 2006, 64 patients were enrolled: 32 patients (26 colon and 6 gastric cancer) were randomly assigned to group A and 32 (23 colon and 9 gastric cancer) to group B. The main patients’ characteristics are shown in Table 1.
Neurotoxicity
All of the enrolled patients were assessed for toxicity. The median number of received cycles of chemotherapy was 12 in both groups; a total of 376 cycles were given to the patients in group A and 367 to those in group B (Table 2). A similar median received dose intensity of l-HOP was reported in both groups. Two patients in group A withdrew from therapy after 9 and 10 cycles because of hematologic toxicity and neurotoxic effects, respectively. Three patients in group B withdrew from therapy after 8–10 cycles because of neurotoxic effects (two cases) and hematologic toxicity (one case).
Neurosensory toxicity was observed in 84.3% of patients of group A and 93.8% of patients of group B. Grade 2 neurotoxicity was observed in seven patients in group A and 13 patients in group B (21.8% vs. 40.6%; P = 0.1). Grade 3 neurotoxicity, with patients displaying difficulties in fine manual activities such as writing and buttoning, occurred after 9 and 10 cycles in two patients of group A, (cumulative dose of l-HOP: 745 and 810 mg/m2, respectively), and after a median cumulative l-HOP dose of 790 mg/m2 (range 765–1,020 mg/m2) in six patients of group B. The difference between the two groups in terms of grade 3 neurotoxicity was not statistically significant (6.2% vs. 18.7%; P = 0.2). If grade 2 was considered together with grade 3, the percentage of patients with grade ≥2 neurotoxicity was statistically lower in group A than in group B (28.1% vs. 59.3%: P = 0.02).
The analysis of the incidence of neurotoxicity in relation to the number of received cycles revealed a statistically lower percentage of cycles with grade ≥2 neurotoxicity in group A (6.1%) than in group B (18.5%) (P < 0.001). The grade ≥2 neurotoxic effects, which consisted mostly of distal cold-related paresthesia, were much more frequent after the first 6 cycles of chemotherapy in both groups. Pharyngolaryngeal disesthesia was not observed in group A, while it was reported in two patients in group B.
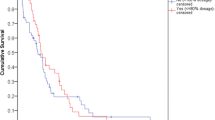
Figure 1 showes the incidence of grade ≥2 neurotoxicity within 6 months after the end of chemotherapy. At 1-month follow-up, grades 2 and 3 neurotoxic effects were observed in 4 and 1 patients in group A and in 6 and 2 patients in group B. At 3-month follow-up, grade 2 neurotoxicity was present in 2 patients in group A and 2 patients in group B, while grade 3 was observed in one patient in group B.
At 6-month follow-up, no patient in group A presented grade 2 or 3 neurotoxicity, while one patient in group B still presented grade 3 neurotoxic effects. In this patient the intensity of neurotoxicity changed from grade 3 to 2 at the 12-month follow-up.
Other treatment-related toxicities.
The other observed toxicities are listed in Table 3. Overall, toxicity was mild, and no statistical significant difference between the two groups was observed.
The most common side effect was neutropenia, but it reached grade 4 in only one patient in group A and 2 patients in group B, and it rapidly recovered with adequate supportive care. Grade 4 diarrhea was not observed. One patient in group A had persistent grade 3 thrombocytopenia and was withdrawn from the study after 9 cycles of chemotherapy. No patient had severe allergic reactions.
Follow-up
At the time of analysis, after a median follow-up of 22 months, four patients in group A (two colon and two gastric), and five patients in group B (one colon and four gastric) had relapsed. One patient in group A and two patients in group B had died.
Discussion
This randomized study assessed the clinical neurotoxicity of two different l-HOP infusion schedules–continuous 6-h infusion (group A) compared with 2-h infusion (group B), in patients with colon and gastric cancer receiving FOLFOX-4 regimen as adjuvant treatment. Although the median received dose intensity of l-HOP and the overall incidence of neurotoxicity were similar in the two groups, there was a trend to less grade 3 neurotoxicity in group A than in group B (6.2% vs. 18.7%) (Table 2). This finding suggests the efficacy of the 6-h continuous infusion of l-HOP in preventing and reducing the acute neurotoxic effects during an adjuvant FOLFOX-4 regimen.
The percentage of ≥2 neurotoxicity observed in group A was apparently lower than that reported by the trial of Giacchetti et al., where the same l-HOP 6-h infusion was applied: 45% of patients with grades 2–3 neurotoxicity, and 13% of patients with moderate functional impairment from peripheral sensory neuropathy [11]. The study of Giacchetti et al. had been performed in metastatic disease, and patients received up to 1,650 mg/m2 of l-HOP administered at single doses of 125 mg/m2; the onset of severe neurotoxicity occurred after a median cumulative l-HOP dose of 1,100 mg/m2. In our adjuvant study patients received a maximum cumulative dose of l-HOP of 1,020 mg/m2, and, moreover, a lower single dose of l-HOP was used (85 mg/m2), which is associated to a lower risk of development of acute neurotoxicity [13, 14].
The incidence of ≥2 neurotoxicity in group B compared well to that reported in the majority of previous studies in which l-HOP was used as the conventional 2-h infusion [1]. A lower incidence of grade of ≥2 neurotoxic effects (44.0%) was reported in the large MOSAIC trial, but that study was not designed to evaluate clinical neurotoxicity, which might be underestimated [2].
Our findings may be explained on the basis of the mechanism of neurotoxicity induced by l-HOP. After administration, l-HOP undergoes biotransformation into total platinum, ultrafilterable or “free” platinum and erythrocyte platinum; the pathologic presence of platinum adducts within the peripheral nerve system is the principle mechanism of l-HOP neurotoxicity [15]. The acute neurotoxicity of l-HOP is not explained by morphologic damage of the nerve, but it has been referred to as “acute channelopathy,” characterized by an increased excitability of nerve and muscle cells [16]. Therefore, the reduction in the severity of neurotoxic effects observed in our randomized study was probably due to the prolonged infusion of l-HOP as 6 h, which led to a lower peak plasma concentrations of the drug and therefore to a consequent decrease in nerve–excitability compared to conventional 2-h infusion.
Another point to consider is that prolonging the l-HOP infusion until the middle of the day (from 1,000 to 1,600) may have contributed to improve tolerability of neurotoxic effects, as a result of circadian rhythms [17]. A randomized study demonstrated that the chronomodulated delivery of l-HOP, with a peak rate at 1,600 h, produced less peripheral sensory neuropathy, less diarrhea and less leucopenia than a constant rate infusion of I-HOP for 5 days, and was more effective [18]. However, despite that the preclinical and clinical chronopharmacology of l-HOP has been investigated for many years in an attempt to minimize toxicity, further investigations are needed to clarify the relationships between drug scheduling, pharmacokinetis, pharmacodynamics, and circadian rhythms of l-HOP [19].
Our study showed a statistically significant difference in the number of received cycles with grade ≥2 neurotoxicity between the two groups (6.1% group A vs. 18.5% group B, P < 0.001) (Table 2). This finding may appear particularly important for patients receiving a planned number of 12 FOLFOX-4 cycles, because grade ≥2 paresthesia are discomforting for patients. Therefore, the reduction in the number of cycles affected by grade ≥2 neurotoxicity may allow more patients to better tolerate and accept all the planned adjuvant chemotherapy.
Among other attempts to prevent neurotoxicity from l-HOP, the retrospective analysis of Gamelin et al. suggested that the reduction in incidence and intensity of acute l-HOP symptoms and the delay of cumulative neuropathy might be obtained with Ca gluconate and Mg sulfate 1 g each, infused before the l-HOP administration [20]. However, the analysis was performed in patients receiving a small number of cycles of chemotherapy (from 1 to 6 cycles in most cases), and have to be confirmed by other studies.
Interesting results in terms of prevention of neurotoxicity were reported with the infusion of glutathione (GSH) before l-HOP administration [21]. Although only ten patients in the GSH group received 12 cycles of treatment, the overall incidence of grade 2–4 neurotoxicity was only 9.5% in the GSH group, and 58% in the placebo group. Moreover, in the GSH group there was not a statistical difference in the values of latency and amplitude of the sensory nerve conduction. No neurophysiologic investigation was performed in our population study because the principle end-point was the clinical evaluation of the prevention and reduction of acute neurotoxicity, which is not related to morphologic damage of chemotherapy.
Other recent studies did not support a role for gabapentin, an anti-convulsant agent used for neuropathic pain, in reducing the incidence or severity of l-HOP-induced sensory neurotoxicity [22, 23].
Several authors applied different treatment options in order to prevent neurotoxic effects, such as sequential or alternating regimens, or the stop-and-go strategy, which were useful to minimize toxicity while optimizing the cumulative l-HOP dose, but these treatment options are currently used only in patients with metastatic disease [24–27].
However, it must be remembered that the comparability of published studies is difficult, because neurotoxicity is often graded according to different toxicity criteria scales, and, moreover, the criteria for dose modification and delay of chemotherapy are not always the same in the various trials in which l-HOP is used [20, 21].
In our study, during the 6 months following the end of adjuvant chemotherapy, grade ≥2 neurotoxicity was resolved in the majority of patients in both groups (Fig. 1). This trend was similar to that observed in the MOSAIC trial, where the incidence of grade 3 sensory neuropathy was 12.4% during treatment, and decreased to 1.1% at 1 year of follow-up [2]. Nevertheless, cumulative sensory neuropathy from l-HOP is generally reversible and predictable, and this characteristic is important for the design of all adjuvant studies including this drug. Dysesthesia or paresthesia causing functional impairment usually develops in around 50% of patients at a cumulative dose of 1,170 mg/m2 [7]. Therefore, the low incidence of severe neurotoxicity observed in our population study was mainly due to the maximum cumulative dose of 1,020 mg/m2, which was received by the patients.
The analysis of the other treatment-related toxicities revealed that the safety profile was favorable for both l-HOP schedules (Table 3). The most frequent toxicity was haematological, as expected, and its rate was similar to that observed in other FOLFOX-4 studies.
After a median follow-up of 22 months, four patients in group A and 5 patients in group B relapsed; the assessment of efficacy was not the main end-point of this study, and the small sample size and the short follow-up time prevent us to draw any deduction on disease-free and overall survival.
In conclusion, this study suggests that l-HOP as a continuous 6-h infusion is useful in preventing and reducing acute l-HOP induced neurotoxicity in patients with colon and gastric cancer receiving FOLFOX-4 regimen as adjuvant treatment. Given that a 6 h infusion is very inconvenient for both patients and staff, and has significant health resource utilization implications, a general change in clinical practice should not occur until a second study supports our findings.
References
De Gramont A, Figer A, Seymour M, et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Andrè T, Boni C, Mounedji-Boudiaf L, Navarro M, et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343–2351
Louvet C, Andre T, Tigaud JM, et al (2004) Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol 20:4543–4548
Al-Batran SE, Atmaca A, Hegewisch-Becker S, et al (2004) Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol 22:658–663
Leong T (2005) Chemotherapy and radiotherapy in the management of gastric cancer. Curr Opin Gastroenterol 21:633–635
Wilson RH, Lehky T, Thomas RR, Quinn MG, Floeter MK, Grem JL (2002) Acute oxaliplatin-induced peripheral nerve hyperexcitability. J Clin Oncol 20:1767–1774
Grothey A (2003) Oxaliplatin-safety profile: neurotoxicity. Semin Oncol 4:5–13
Cassidy J, Misset JL (2002) Oxaliplatin-related side effects: characteristics and management. Semin Oncol 29(suppl 15):11–20
Raymond E, Chaney SG, Taamma A, Cvitkovic E (1998) Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol 9:1053–1071
Extra JM, Marty M, Brienza S, Misset JL (1998) Pharmacokinetics and safety profile of oxaliplatin. Semin Oncol 25:13–22
Giacchetti S, Perpoint B, Zigani R, et al (2000) Phase III multicenter randomised trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 18:136–147
Gamelin E, Gamelin L, Bossi L, Quasthoff S (2002) Clinical aspects and molecular basis of oxaliplatin neurotoxicity: current management and development of preventive measures. Semin Oncol 29(suppl 15):21–33
Maindrault-Goebel F, de Gramont A, Louvet C, et al (2001) High-dose intensity oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX7). Eur J Cancer 37:1000–1005
Goldstein D, Mitchell P, Michael M, et al (2005) Australian experience of a modified schedule of FOLFOX with high activity and tolerability and improved convenience in untreated metastatic colorectal cancer patients. Br J Cancer 92:832–837
Kweekel DM, Gelderblom H, Guchelaar H-J (2005) Pharmacology of oxaliplatin and the use of pharmacogenomics to individualize therapy. Cancer Treat Rev 31:90–105
Moxley RT III (2000) Channellopathies. Curr Treat Options Neurol 2:31–47
Bruguerolle B (1998) Chronopharmacokinetics: current status. Clin Pharmacokinet 35:83–94
Levi F, Zidani R, Misset JL, et al (1997) Randomized multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet 350:681–686
Levi F, Misset JL, Brienza S, et al (1992) A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal cancer. Cancer 69:893–900
Gamelin L, Boisdron-Celle, Delva R, et al (2004) Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res 10:4055–4061
Cascinu S, Catalano V, Cordella L, et al (2002) Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomised, double-blind, pacebo-controlled trial. J Clin Oncol 20:3478–3483
Wong GY, Michalak JC, Sloan JA, et al (2005) A phase III double blinded, placebo controlled, randomized trial of gabapentin in patients with chemotherapy-induced peripheral neuropathy: a north central cancer treatment group study. J Clin Oncol 23(Suppl 16):Abstract 8001
Mitchell PL, Goldstein D, Michael M, et al (2006) Addition of gabapentin to a modified FOLFOX regimen does not reduce oxaliplatin-induced neurotoxicity. Clin Colorectal Cancer 6:146–151
Petrioli R, Sabatino M, Fiaschi AI, et al (2004) UFT/leucovorin and oxaliplatin alternated with UFT/leucovorin and irinotecan in metastatic colorectal cancer. Br J Cancer 90:306–309
Tournigand C, Cervantes A, Figer A, et al (2006) OPTIMOX1: a randomized study of FOLFOX 4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer- a GERCOR study. J Clin Oncol 24:394–400
Maindrault-Goebel F, Lledo G, Chibaudel B, et al (2006) OPTIMOX2, a large randomised phase II study of maintenance therapy or chemotherapy-free intervals (CFI) after FOLFOX in patients with metastatic colorectal cancer (MRC). A GERCOR study ASCO 24:3504
Petrioli R, Paolelli L, Marsili S, et al (2006) FOLFOX-4 stop and go and capecitabine maintenance chemotherapy in the treatment of metastatic colorectal cancer. Oncology
Acknowledgments
We thank Dr. Stefania Rossi for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrioli, R., Pascucci, A., Francini, E. et al. Neurotoxicity of FOLFOX-4 as adjuvant treatment for patients with colon and gastric cancer: a randomized study of two different schedules of oxaliplatin. Cancer Chemother Pharmacol 61, 105–111 (2008). https://doi.org/10.1007/s00280-007-0454-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0454-3