Abstract
Trastuzumab has had an enormous impact on the clinical management of breast cancer: the survival of Her-2-positive metastatic breast cancer patients has improved significantly and tumor Her-2 status has been built into the decision-making tree for primary breast cancer patients. Several pioneering studies have shown that trastuzumab-combined chemotherapy elicits high levels of pathological complete response in the neoadjuvant setting. Currently, therefore, a more precise understanding of the mechanisms of therapeutic response is needed so that trastuzumab-based therapies can be optimized more individually. It might also be important to investigate, with greater depth, the interaction between the Her-axis and the hormone-axis. This short review describes and discusses these topics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-Her therapy is a good model to show how molecular-targeting therapy (MTT) has been developing (both preclinically and clinically), what it is effective against and where this technology is headed. Trastuzumab, a humanized anti-Her-2 monoclonal antibody, has, without doubt, been remarkably successful. Several points can be raised to explain why trastuzumab MTT is exceptionally fruitful. First, a highly specific receptor protein for breast cancer growth was chosen as the molecular target. In addition, the amount of extracellular Her-2 expression of Her-2-positive cancer cells greatly differs from that of normal host cells, which lends significant tumor selectivity. Second, the therapeutic strategy, particularly in combination with other therapeutic modalities, was designated based on carefully obtained experimental results and systematically planned clinical trials [16]. Third, the methodology for Her-2 testing has been standardized globally. It is also important to note that severe adverse effects, such as cardiac toxicity, were taken care of promptly and appropriately. Currently, the main aim is to realize further therapeutic optimization and enhancement of treatment using trastuzumab. In this article, recent improvements of survival, yielded by trastuzumab-based therapy in Her-2-positive metastatic breast cancer patients, have been summarized and several translational research aspects for future anti-Her-2 therapy have been touched upon.
Treatment algorithm for Her-2-positive recurrent or advanced breast cancer
Since the incorporation of trastuzumab in metastatic breast cancer treatment, the algorithm shown in Fig. 1 has been used. Trastuzumab-containing therapy is first-line therapy for life-threatening diseases. Trastuzumab is also indicated as first-line therapy in Her-2-positive and hormone receptor-negative cases. For hormone receptor-positive cases, whose disease is non-life threatening, hormone therapy is considered as initial treatment, and treatment may be switched to trastuzumab-containing regimens if progression occurs. As chemotherapy combinations, taxanes or vinorelbine and then capecitabine have been used in the majority of cases. Capecitabine has also been applied to second- and third-line combination therapies. Trastuzumab has been continuously used, right until the time the disease enters the end stage, unless a severe adverse event emerges. For example, in patients who develop brain metastasis while on trastuzumab therapy, surgical removal or irradiation treatments are prioritized, but trastuzumab is continued wherever possible. Cardiac function is monitored by echocardiograms and cardiac pool scintigraphy periodically, and treatment is stopped if severe cardiotoxicity arises. No anthracycline has been combined with trastuzumab in our series. Tumor Her-2 status is examined in formalin-fixed paraffin-embedded tumor tissues, and the 3+, scored by immunohistochemistry and fluorescence in situ hybridization (FISH)-positive cases, are determined as Her-2-positive.
Survival outcome in Her-2-positive metastatic breast cancer
At our institution, a 3-year survival rate among Her-2-positive metastatic breast cancer patients has reached >50% (Fig. 2). The longest response (>5 years) was achieved in a 35-year-old patient who had multiple liver metastases. She underwent trastuzumab therapy continuously for this duration. No significant survival difference has been observed between patients with life-threatening cancers and those with non-life-threatening cancers. When compared with historical data from the same category of Her-2-positive metastatic breast cancer patients, who were treated by non-trastuzumab-containing regimens from 1995 to 2000, survival improvements in recent years is very clear – a 50% survival rate: 12.5 months in patients treated from 1995 to 2000 vs. >36 months in those treated from 2000 to 2004. Although many other factors, including the launch of new agents other than trastuzumab might be involved, the biggest impact seems to be that of trastuzumab, since no similar large survival improvement has been observed in Her-2-negative metastatic breast cancer patients.
Survival curve of Her-2-positive breast cancer patients, treated by trastuzumab-containing therapies, at Tokyo Metropolitan Cancer and Infectious Disease Center, Komagome Hospital since 2000 (n=55). Her-2 status was determined by Hercept test and FISH test (A). Survival curves stratified by the presence and absence of life-threatening disease. No significant difference was observed (B)
Trastuzumab for primary breast cancer
The action of trastuzumab in primary breast cancer patients is currently being tested in several clinical trials. In the postoperative adjuvant setting, four ongoing [13] large-scale clinical trials are assessing the synergy between trastuzumab and paclitaxel q3 or q1, the clinical potency of a combination of trastuzumab with platinum and docetaxel, which has been characterized as the most potent combination experimentally, and the impact of the extension of trastuzumab treatment durations. The core survival results will come out in the next few years. In the preoperative setting, various combinations, with different chemotherapeutic agents, are also being examined. Recently, Buzdar et al. [3] reported a significant 39% gain in the pathological complete remission (pCR) rate by adding trastuzumab to four cycles of paclitaxel, followed by four cycles of fluorouracil, epirubicin, and cyclophosphamide sequential combination chemotherapy. The reported pCR rate looks far higher than any other pCR rates from trials conducted in Her-2 unselected populations [18]. Since recent neoadjuvant chemotherapy trials have confirmed that pCR is a reliable surrogate marker for long-term favorable prognosis, it is highly likely that trastuzumab-combined therapy confers survival benefit advantages in these pCR cases [19]. Moreover, theoretically, it seems that many potential non-pCR cases for trastuzumab therapy are excluded by Her-2 selection. Among the selected population, trastuzumab might be highly effective (Fig. 3).
Mechanisms of therapeutic response of trastuzumab
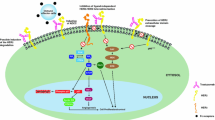
To expand its efficacy, it is necessary to know more about how trastuzumab elicits a therapeutic response. Very little is known about its precise mechanisms. Many investigations have confirmed that trastuzumab potentiates the efficacy of other types of therapy, in particular chemotherapy, by facilitating the induction of apoptosis as a sensitizer [16]. The apoptosis index was shown to have increased surprisingly, by a combination use in experimental animal models. This synergistic effect was confirmed in clinical trials, wherein significant survival prolongation was demonstrated by adding trastuzumab onto the basal chemotherapy [7]. As a different aspect of the therapeutic response mechanism, Jain’s group reported that trastuzumab was able to normalize and regress tumor neovasculature in Her-2-overexpressing human breast tumors in mice, by modulating the effects of multiple pro- and anti-angiogenic factors [12]. It has also been reported, from other investigators, that Her-2 blockade downregulates expression of the vascular endothelial growth factor, a central player of tumor angiogenesis, in vivo [20]. Of next interest, is the examination of what emerges on human tumor vasculature, after trastuzumab treatment. The immunological aspect is also crucial to understand trastuzumab’s therapeutic response mechanism in vivo. Recently, Kono et al. demonstrated that Her-2-overexpressing gastric cancer cells are killed by trastuzumab-mediated antibody-dependent cell-mediated cytotoxicity (ADCC), and that trastuzumab-induced ADCC is significantly correlated with the degree of Her-2 expression on these cancer cells [15]. In addition, trastuzumab-mediated ADCC activity was shown to depend on natural killer (NK) cell function. Poorly functioning NK cells from advanced-disease patients display impaired trastuzumab-mediated ADCC; whereas NK cells from healthy individuals and from patients with early disease, do not. According to these findings, it might be possible to hypothesize that trastuzumab can achieve higher responses in early-stage primary breast cancer patients than in advanced metastatic cancer patients. In a recent pilot study that looked at the molecular pathological changes caused by trastuzumab in the neoadjuvant treatment of primary breast cancer, a strong infiltration of lymphoid cells was observed in all cases despite there being no detection of the downmodulation of Her-2, changes in vessel diameters and changes of proliferation [8]. The importance of ADCC is also indicated for various other types of cancers, including ovarian and uterine cancer [6, 9, 17]. Additionally, several new approaches for potentiating trastuzumab-mediated ADCC, by means of immune modulators, are under investigation [1].
Prediction of therapeutic response for trastuzumab
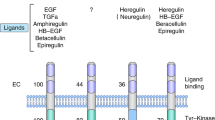
Although such cases are not in the majority, it has been noted that some tumors do not show any response to trastuzumab and some respond only minimally. These spontaneous therapeutic resistances might be due to the original tumor phenotype. Recent studies have clarified that the dissociation speed of the ligand differs between Her-1/Her-2 hetero-dimerization and Her-1/Her-1 homo-dimerization [5]. Transmission speed and potency are also influenced by receptor dimerization status (Fig. 4). Her family receptor dimerization status is extremely diverse in primary breast tumors [10]. Thereby, many new technologies and platforms are under development for the purpose of quantitatively analyzing these features in human tumor materials [4].
From an immunological aspect, it might be reasonable to measure and monitor NK cell function, so as to predict therapeutic responses and resistances. It is well known that the natural cytotoxic activity of peripheral-blood lymphocytes differs among healthy individuals, and that these differences are related to the risk of breast cancer occurrence [11]. In cancer patients, in general, NK cell activity is downregulated in proportion to disease progression; however, the degree of decrease differs according to disease status and the individual’s condition. NK cell activity might be altered not only by cancer stress, but also by cancer treatment-associated stress, such as adverse events. Immunological studies might deliver novel insights for the prediction of responses to trastuzumab therapy.
Influence of other treatments on Her-2 axis in breast cancer
Recently, Zhu et al. [21] reported an intriguing finding that Her-2 expression is downregulated by aromatase inhibition in Her-2-positive and hormone receptor-positive primary breast cancer. Both Her-2 protein expression and the ratio of Her-2 gene-amplified cases decreased significantly. These events seem more likely to occur in responders to aromatase inhibition than in non-responders. Although the mechanism is still unclear, trastuzumab for the treatment of hormone-dependent breast cancer, in conjunction with aromatase inhibition, is a noteworthy concept. Several studies have shown significant inverse correlation between Her-2 expression and the hormone receptor expression in human breast cancer [14]. The estrogen blockade is capable of upregulating estrogen receptor expression; additionally, it is also possible to downregulate Her-2 expression. Therefore, one might be able to hypothesize that estrogen controls the expression balance between hormone receptors and Her-2 in human breast cancer tissues (Fig. 5). Indeed, a recent study showed that Her-2 gene amplification was rare in male breast cancer, which is a remarkable contrast [2]. It might be interesting to learn the Her-2 status of primary breast cancer patients enrolled in chemoprevention trials of aromatase inhibitors.
Conclusion
Trastuzumab has brought significant advantages for Her-2-positive breast cancer patients and promises to bring further benefits for future cancer patients. Trastuzumab is an interesting tool with which to understand human cancer biology. Investigating the mechanisms of its therapeutic response might give us some hints as to how cancer cells develop continuous growth and how these cells escape systemic surveillance. Further clinical trials and translational research may provide many exciting insights.
References
Adams S, Miller GT, Jesson MI, Watanabe T, Jones B, Wallner BP (2004) PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism. Cancer Res 64:5471–5480
Barlund M, Kuukasjarvi T, Syrjakoski K, Auvinen A, Kallioniemi A (2004) Frequent amplification and overexpression of CCND1 in male breast cancer. Int J Cancer 111:968–971
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23:3656–3659
Chan-Hui PY, Stephens K, Warnock RA, Singh S (2004) Applications of eTag trademark assay platform to systems biology approaches in molecular oncology and toxicology studies. Clin Immunol 111:162–174
Citri A, Skaria KB, Yarden Y (2003) The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res 284:54–65
Clynes RA, Towers TL, Presta LG, Ravetch JV (2000) Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor target. Nat Med 6:443–446
Finn RS, Slamon DJ (2003) Monoclonal antibody therapy for breast cancer: herceptin. Cancer Chemother Biol Response Modif 21:223–233
Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, Oliviero B, Ballardini B, Da Prada G, Zambelli A, Costa A (2004) Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res 10:5650–5655
Hellstrom I, Goodman G, Pullman J, Yang Y, Hellstrom KE (2001) Overexpression of HER-2 in ovarian carcinomas. Cancer Res 61:2420–2423
Hudelist G, Singer CF, Manavi M, Pischinger K, Kubista E, Czerwenka K (2003) Co-expression of ErbB-family members in human breast cancer: Her-2/neu is the preferred dimerization candidate in nodal-positive tumors. Breast Cancer Res Treat 80:353–361
Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K (2000) Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 356:1795–1799
Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK (2002) Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 416:279–280
Jones RL, Smith IE (2004) Efficacy and safety of trastuzumab. Expert Opin Drug Saf 3:317–327
Konecny G, Pauletti G, Pergam M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M, Wang HJ, Beryt M, Seshadri R, Hepp H, Slamon DJ (2003) Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst 95:142–153
Kono K, Takahashi A, Ichihara F, Sugai H, Fujii H, Matsumoto Y (2002) Impaired antibody-dependent cellular cytotoxicity mediated by herceptin in patients with gastric cancer. Cancer Res 62:5813–5817
Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ (2004) Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 96:725–727
Santin AD, Bellone S, Gokden M, Palmieri M, Dunn D, Agha J, Roman JJ, Hutchins L, Pecorelli S, O’Brien T, Cannon MJ, Parham GP (2002) Overexpression of HER-2/neu in uterine serous papillary cancer. Clin Cancer Res 8:1271–1279
Toi M, Bando H, Chow LW (2004) Novel insights in clinical trial with preoperative systemic therapy for primary breast cancer. Biomed Pharmacother 58:531–535
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 30:96–102
Yokoi A, McCrudden KW, Huang J, Kim ES, Soffer SZ, Frischer JS, Serur A, New T, Yuan J, Mansukhani M, O’Toole K, Yamashiro DJ, Kandel JJ (2003) Blockade of Her2/neu decreases VEGF expression but does not alter HIF-1 distribution in experimental Wilms tumor. Oncol Rep 10:1271–1274
Zhu L, Chow LW, Loo WT, Guan XY, Toi M (2004) Her2/neu expression predicts the response to antiaromatase neoadjuvant therapy in primary breast cancer: subgroup analysis from celecoxib antiaromatase neoadjuvant trial. Clin Cancer Res 10:4639–4644
Acknowledgements
This work was supported by a grant for Optimization of new anticancer drugs from the Health and Labor Science Research Grants of Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labor and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toi, M., Horiguchi, K., Bando, H. et al. Trastuzumab: updates and future issues. Cancer Chemother Pharmacol 56 (Suppl 1), 94–99 (2005). https://doi.org/10.1007/s00280-005-0110-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-0110-8