Abstract
Background: The rationale supporting the use of intraperitoneal chemotherapy in peritoneal surface malignancy relates to a large local–regional effect and low systemic toxicity. While optimizing the use of this treatment strategy, little information regarding the effect of volume of chemotherapy solution is available. Objective: The goal of this study was to provide data regarding the effect of volume of chemotherapy solution on the pharmacokinetics of intraperitoneal chemotherapy. Data by which to optimally adjust this parameter during intraperitoneal chemotherapy treatments were sought. Methods: Forty-eight patients with peritoneal surface malignancy were treated with hyperthermic intraperitoneal mitomycin C chemotherapy after a complete cytoreduction to remove all visible evidence of mucinous tumor. The dose of mitomycin C was always 12.5 mg/m2 in males and 10 mg/m2 in females. The first 12 patients were treated with 6 l of 1.5% dextrose peritoneal dialysis solution. The next 14 patients were treated with 4 l of fluid and then ten patients were treated with 2 l. In the last 12 patients the volume of fluid was 1.5 l/m2 . Blood, peritoneal fluid, and urine samples were obtained every 15 min for 90 min; additional blood and urine samples were obtained at 120 min. Mitomycin C concentrations, urine volumes, and final intraperitoneal fluid volume were obtained. Results: The intraperitoneal and the plasma concentrations were highest in the 2-l group, less in the 4-l group, and least in the 6-l group. All differences were statistically significant. Also, the percent of mitomycin C absorbed decreased significantly from 2, to 4, to 6 l of fluid. The area under the curve (AUC) ratio of intraperitoneal concentration times time to intravenous concentration times time was 27.01±4.92 for 2 l, 22.22±7.95 for 4 l, and 24.01±8.46 for 6 l. These differences were not statistically significant. If both the volume of chemotherapy solution and the total dose of mitomycin C were determined from the body surface area, the pharmacokinetics of intraperitoneal mitomycin C were more consistent. Conclusions: In order to prescribe a uniform treatment for patients receiving hyperthermic intraperitoneal mitomycin C, the total dose of the drug and the total volume of chemotherapy solution should be determined from the body surface area. If the volume of chemotherapy solution is not based on patient body surface area, predictions regarding toxicity are less precise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A survey of the recent medical literature suggests an increased number of clinical studies regarding the use of intraperitoneal chemotherapy in the management of gastrointestinal cancer. In a majority of studies the chemotherapy delivery was a planned part of the surgical procedure with administration in the operating theater with heat or during the first few postoperative days. Yu et al. performed a phase III study in 248 gastric cancer patients who had a complete resection of the primary cancer [1, 2] The survival of patients who had gastrectomy plus early postoperative intraperitoneal chemotherapy was superior to that of patients having gastrectomy alone (P=0.0278). The stage III patients seemed to profit the greatest (P=0.0024). Verwaal and colleagues performed a phase III study with patients who had carcinomatosis from colorectal cancer. Patients who had cytoreduction plus hyperthermic intraoperative intraperitoneal mitomycin C chemotherapy had a median survival of 22.3 months as compared to 12.6 for patients treated by palliative surgery plus systemic chemotherapy (P=0.032) [3]. A registry report of 506 patients with carcinomatosis from colorectal cancer showed a 5-year survival of 19% and a median survival of 19.2 months [4]. A recent review suggested a prominent role for perioperative intraperitoneal chemotherapy for both prevention and treatment of carcinomatosis from gastrointestinal cancer [5, 6].
The most favorable results in the treatment of carcinomatosis are with patients who have dissemination of appendiceal malignancy [7, 8]. The combined approach to treatment involves cytoreductive surgery to remove visible disease and perioperative intraperitoneal chemotherapy to eradicate minimal residual disease. A less invasive disease process, the redistribution of mucinous tumor away from the small bowel, and a low incidence of liver and lung metastases make complete visible surgical eradication of appendiceal carcinomatosis possible in over two-thirds of patients. The 10-year disease-free survival in appendiceal epithelial tumors with minimally aggressive pathology is above 80% in patients who had a complete cytoreduction. No long-term disease-free survival in the absence of this new approach has been reported. These patients have become ideal candidates for treatment by intraperitoneal chemotherapy using mitomycin C; currently, other treatment strategies such as serial debulking may be considered suboptimal. However, the technology for drug delivery is not uniform and unpredicted severe toxicity occurs keeping the morbidity and mortality of this treatment strategy high. Dose of drug, total time for administration, and heat tolerance have been studied with some agreement regarding optimal use. However, little prior information regarding the effect of the volume of chemotherapy solution on pharmacokinetics of intraperitoneal mitomycin C chemotherapy is available. The goal of this study was to provide information regarding the pharmacokinetic changes in intraperitoneal mitomycin C delivery that occurs with changes in the volume of fluid.
Methods
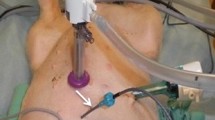
Forty-eight patients who had mucinous peritoneal carcinomatosis from appendiceal malignancy were treated. All patients had the disease histopathology and its intraperitoneal dissemination confirmed preoperatively by biopsy and abdominopelvic CT. All patients were required to have a complete visible removal of tumor by peritonectomy procedure [9]. Following the tumor resection but prior to intestinal reconstruction and abdominal closure, the skin edges were elevated on a retractor to create a reservoir within the abdomen and pelvis [10]. One inflow catheter and four outflow catheters were positioned. These tubes were connected to a heater circulator and a heat exchanger so that the mitomycin C chemotherapy solution had a target temperature of 43°C at the inflow catheter and 40.0°C at the pelvic outflow drains. The inflow catheter was routinely placed in the right upper quadrant between the right hemidiaphragm and the right lobe of the liver. The space above the open abdominal incision was partially uncovered to allow access of the surgeon’s double-gloved hand. The warm chemotherapy solution was manually distributed intermittently throughout the 90 min of treatment to maintain uniform heat and chemotherapy distribution.
The chemotherapy was always diluted in a 1.5% dextrose peritoneal dialysis solution (Abbott, Abbott Park, IL, U.S.A.). In these studies all chemotherapy solution was infused into the peritoneal cavity within 6 min. Flow was approximately 1 l/min throughout the 90-min treatment. All chemotherapy solution (2, 4, 6 l or 1.5 l/m2) was within the peritoneal cavity at all times. The reservoir in the irrigation system was always kept as empty as possible. Preliminary studies with peritoneal dialysis solution alone determined that 6 l was the maximal volume that could be retained in the abdominopelvic reservoir.
At pre-treatment and at 15-min intervals during the 90-min treatment, a 3-ml sample of blood, peritoneal fluid, and urine were obtained. An additional sample of blood and urine was obtained at 120 min. The final volume of intraperitoneal chemotherapy solution was recorded at the completion of the treatment. In order to calculate the absorption of mitomycin C during hyperthermic intraoperative intraperitoneal chemotherapy treatments, the chemotherapy solution volume was assumed constant at 2, 4, or 6 l. Also, the volume of urine was recorded for each 15-min interval for 120 min.
Blood samples were centrifuged and the plasma was separated and transferred to polypropylene vials for storage. Perfusate samples were also centrifuged, to remove any blood components, and the supernatant fluid was transferred to polypropylene vials. All samples were stored frozen at −20°C. Samples were thawed as a batch for analysis by high-pressure liquid chromatography (HPLC). Each batch of samples was assayed within 24 h after the 120-min samples were collected.
The HPLC technique was similar to that described elsewhere [11, 12]. Briefly, isolation of mitomycin C from plasma was by solid-phase extraction. Perfusate and urine samples which contained relatively high concentrations of the drug were appropriately diluted with water and filtered through 0.2-μm nylon syringe filters for HPLC injection.
The HPLC system consisted of a Shimadzu LC7A instrument equipped with SPD-6AV (UV–vis) detector (set at 365 nm for mitomycin C), along with a C-R6A Chromatopac data processor. Reversed-phase columns (250×4.6 mm of 300 A 5-μm silica) bonded to C18 (Dynamax; Rainin Instruments, Emeryville, CA, USA) were used, coupled to a guard column of the same chemical consistency. The mobile phase consisted of methanol–water (3:7) for mitomycin C run isocratically at 0.9 ml/min. Sample injections were 50 μl. All solvents were Fisherbrand, HPLC grade (Fisher Scientific, Norcross, GA, USA).
Clinical data collected in order to compare the four groups of patients include age, gender, body surface area, median temperature at the inflow site, urine output over 90 min, tumor volume as assessed by the peritoneal cancer index, number of peritonectomy procedures, and tumor histopathology.
The amount of tumor present as determined at the time of abdominal and pelvic exploration was estimated by the peritoneal cancer index. To arrive at a score, the size of the intraperitoneal nodules was assessed. The lesion-size (LS) score was used. An LS-0 score indicated that no malignant deposits are visualized. An LS-1 score indicated the presence of tumor nodules less than 0.5 cm. The number of nodules was not scored; only the size of the largest nodules was scored. An LS-2 score indicates the presence of tumor nodules between 0.5 and 5.0 cm. LS-3 indicates the presence of tumor nodules greater than 5.0 cm in any dimension. If there was a confluence of tumor, the lesion size was scored as 3.
In order to assess the distribution of peritoneal surface disease, the abdominopelvic regions were utilized. For each of these 13 regions, an LS score was determined. The summation of the LS score in each of the 13 abdominopelvic regions was the Peritoneal Cancer Index (PCI) for that patient. A maximal score was 39 (13×3) [13].
The extent of surgery was estimated by the number of peritonectomy procedures performed. There were eight different procedures each having a score of one. These were total anterior parietal peritonectomy, greater omentectomy and splenectomy, left upper quadrant peritonectomy, right upper quadrant peritonectomy, rectosigmoid colon resection with pelvic peritonectomy, right colectomy, cholecystectomy with lesser omentectomy, and total gastrectomy. One procedure, namely, total abdominal colectomy with pelvic peritonectomy was scored as two peritonectomy procedures [9]. The tumor histopathology was determined to be adenomucinosis, intermediate type or mucinous adenocarcinoma [14].
Patients were selected for a 2-, 6-, or 4-l group in consecutive order. That is, ten patients were treated with 2 l and then an additional ten patients were treated with 6 l, and finally ten patients with 4 l. When data on these three groups were complete, patients were treated with 1.5 l/m2 . An exception to this was our desire to have the same number of male and female patients within each group in order to minimize any differences in the male/female dose of mitomycin C. Another exception occurred when a patient, because of limited abdominopelvic volume, could not receive the entire 6 l. These patients with insufficient abdominopelvic space were not entered into the study or were treated with 4 l of chemotherapy solution. The original plan was not to use the size of the patient or the extent of the surgery to select patients for the pharmacologic groups.
All data were recorded and stored in a Microsoft Excel database. Data points are presented as average ± one standard deviation. The area under the curve (AUC) ratio of peritoneal concentration times time to plasma concentration times time was determined using “Prism for Windows” version 3.0 (GraphPad Software, San Diego, CA, USA). The statistical package SPSS for Windows (SPSS-11.5, Chicago, IL, USA) was used to determine chi-square differences and P values.
Results
Clinical characteristics and treatment variables
The clinical characteristics and treatment variables in all groups of patients are shown in Table 1. No significant differences in age, gender, body surface area, median temperature at the inflow catheter during treatment, urine output during treatment, the number of peritonectomy procedures, and the tumor histology were found. In comparison of PCI between groups, there was a statistically significant increase in the group treated with 6 versus 2 l (P=0.017) and 6 versus 4 l (P=0.0002).
Peritoneal and plasma mitomycin C concentrations
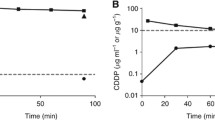
The mean concentrations of mitomycin C in peritoneal fluid ± one standard deviation for patients receiving 2, 4, or 6 l are shown in Fig. 1. The 2-l group had the greatest concentration of drug and the 6-l the least. The differences in these lines were near equidistant during the 90 min of treatment. All three lines are statistically significantly different.
Also shown in Fig. 1 are the concentrations of mitomycin C in the plasma in these three groups of patients. These plasma levels are greatest in the 2-l group, less in the 4-l group, and least in the 6-l group. All three lines are statistically significantly different.
Absorption of mitomycin C from peritoneal fluid to plasma
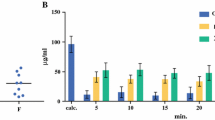
At the conclusion of the 90 min of treatment, the concentration of mitomycin C in peritoneal fluid and the total volume of chemotherapy solution were determined. From these data the total absorption of mitomycin C from the peritoneal fluid to plasma could be determined. In the 2-l group, 82(±4.4)% was cleared from the peritoneal cavity, 72.2(±3.9)% from the 4-l group, and 55.7(±9.8)% from the 6-l group (Fig. 2). All comparisons of these differences were statistically significant (P<0.0001).
Area under the curve ratio
The peritoneal fluid concentration times time divided by the plasma concentration times time was used to construct an area under the curve ratio. This was 27±4.92 for the group treated with 2 l of chemotherapy solution, 22.2±7.95 for 4 l, and 24±8.46 for 6 l. There were no statistically significant differences between these three groups (Fig. 3).
Dosimetry by body surface area for mitomycin C and the carrier solution
In a fourth group of patients, the dose of mitomycin C and the volume of carrier solution were both determined by the patient’s body surface area. As in prior treatments, the dose of mitomycin C remained at 10 mg/m2 for females and 12.5 mg/m2 for males. The volume of chemotherapy solution was 1.5 l/m2 . Figure 4 shows the intraperitoneal and plasma mitomycin C concentrations for these 12 patients.
Discussion
These data demonstrate the need for a volume of chemotherapy solution that is determined by the patients‘ body surface area. The volume should not be determined by the amount of fluid that the administration machine requires for priming. It should not be determined by the amount of fluid required to fill the peritoneal cavity. The pharmacokinetics and therefore the toxicity of intraperitoneal chemotherapy depend not only on dose of drug but also on the volume of chemotherapy solution.
The most reliable assessment of volume effects on the utility of intraperitoneal chemotherapy would be obtained with the use of treatment-related toxicities and survival as endpoints. In this group of patients, approximately 80% of 10-year survival is expected [8]. The mortality is 1.5% and the grade III/IV morbidity 27% [15]. Statistically significant alterations in these clinical data would be difficult and probably impossible to demonstrate. Another reliable parameter to measure would be the amount of chemotherapy present within cancer nodules. Since there were no macroscopic cancer deposits remaining in these patients, this was not possible. Also, the accurate determination of mitomycin C content of tissue has not been technically possible as a result of inextricable binding of this drug to tissue proteins. In the absence of these clinical parameters this study selected pharmacokinetic parameters to estimate the effects of volume on drug efficacy and toxicity. Although these data can only influence the theory of drug delivery, they may be of help in the selection of optimal treatment plans for patients with peritoneal surface malignancy.
In the past it has been very difficult to explain how some groups could use doses of mitomycin C between 30 and 40 mg/m2 and have approximately the same morbidity and mortality of groups that use between 10 and 20 mg/m2 [16]. This manuscript clearly shows that the absorption of drug into the plasma compartment is in large part dependent upon the volume of carrier solution. If a perfusion technique used 6 or more liters of carrier solution, for a given dose of mitomycin C the toxicities would be markedly diminished as compared to the use of 3 l of solution. However, the dose intensity of the local–regional treatment would be greatly reduced. Intraperitoneal chemotherapy treatments can only be compared when both the dose of drug and the volume of carrier solution are considered.
These data support a diffusion model for chemotherapy exit from the peritoneal cavity. The three different concentrations of chemotherapy leave the peritoneal cavity in a linear fashion. The differences in the concentrations in the three groups of patients remain similar over the 90 min. The amount of drug present seems to be controlled by the concentration difference between peritoneal cavity and the plasma.
Two liters was selected as the smallest volume of chemotherapy solution. A lesser volume of fluid would not immerse the entire small bowel and would not result in a uniform treatment. Six liters was the largest volume of chemotherapy solution that could be used without spillage over the skin edges. Four liters was an intermediate amount used to allow for comparisons between groups and to establish trends. As a result of these data, we selected 1.5 l/m2 as the ideal volume; in our treatment methodology this was a volume that covered all small bowel and yet did not result in spillage. These data show that a prescription for perioperative intraperitoneal chemotherapy cannot be considered complete by indicating only the dose of a drug. The drug effects that are expected at the peritoneal surface and in the plasma depend not only on the drug dose but also on the volume of intraperitoneal fluid.
These data support those of Elias and Sideris. They used 2 or 2.5 l/m2 of 5% dextrose carrier solution for a standard dose of oxaliplatin of 410 mg/m2 . The plasma oxaliplatin decreased from 14.1 to 9.1 μg/ml with this decreased concentration of intraperitoneal chemotherapy solution [17].
In conducting this study it was not possible to randomly assign patients to the 2-, 4-, or 6-l group. Patients were placed in the group in consecutive order of ten patients per group. An exception was a few patients in the 6-l group. Some patients who did not have a large volume of mucus ascites causing marked abdominal distention could not accommodate 6 l of carrier solution. As might be expected, more patients in the 6-l group had a larger tumor volume by PCI prior to the cytoreduction (Table 1). Even with tumor volume as an additional variable, the trend of differences between groups was consistent. We suggest that the large differences in the percentage of mitomycin absorbed were caused predominantly by the differences in carrier solution. Ideally, randomization of patients to 2 versus 4 versus 6 versus 1.5 l/m2 would have been a more perfect study. However, within the context of the clinical availability of the patients suitable for treatment, this was thought to be impossible.
A possible criticism of this work would relate pharmacologic differences not to volume but to a different extent of peritonectomy between the four groups of patients. There was a statistically significant increase in the peritoneal cancer index in the group that had 6 l of fluid. However, the extent of peritonectomy is not a likely explanation for these differences in pharmacology; they are most likely related to the differences in volume. Our previous work has demonstrated that the extent of peritonectomy has little if any effect upon intraperitoneal pharmacology [18].
The decisions required to formulate a chemotherapy treatment that occurs as a planned part of a major surgical procedure is a demanding process. The information that is available is not adequate to apply an optimal treatment strategy in all patients. The combined treatment of cytoreductive surgery plus perioperative chemotherapy carries with it 1–8% mortality and a 20–65% morbidity. A large part of the adverse events can be related to the extensive surgical dissection and a requirement for intestinal suture lines. However, perioperative chemotherapy has been shown to slow wound healing, cause bone marrow suppression, and may cause pancreatitis. All of these adverse events can be expected to increase in frequency as the exposure to chemotherapy in the plasma and in the peritoneal cavity increases. Toxicities within the peritoneal cavity such as small bowel fistula and anastomotic leakage undoubtedly increase as the area under the curve drug dose increases. An accurate prescription of intraperitoneal chemotherapy that can predict maximal effect on cancer nodules and minimal toxicity require careful attention to the volume of chemotherapy solution.
References
Yu W, Whang I, Suh I, Averbach A, Chang D, Sugarbaker PH (1998) Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg 228:347–354
Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH (2001) Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg 25:985–990
Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FAN (2003) Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 21:3737–3743
Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, Gutman M, Tentes AAK, Lorimier G, Bernard JL, Bereder JM, Porcheron J, Gomez-Portilla A, Shen P, Deraco M, Rat P (2004) Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer. A multi-institutional study. J Clin Oncol 22:3284–3292
Sugarbaker PH (2004) Managing the peritoneal surface component of gastrointestinal cancer. Part 1. Patterns of dissemination and treatment options. Oncology (Huntingt) 18:51–59
Sugarbaker PH (2004) Managing the peritoneal surface component of gastrointestinal cancer. Part 2. Perioperative intraperitoneal chemotherapy. Oncology (Huntingt) 18:207–219
Sugarbaker PH, Jablonski KA (1995) Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg 221:124–132
Sugarbaker PH (1999) Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol 6:727–731
Sugarbaker PH (1995) Peritonectomy procedures. Ann Surg 221:29–42
Sugarbaker PH, Averbach AM, Jacquet P, Stephens AD, Stuart OA (1996) A simplified approach to hyperthermic intraoperative intraperitoneal chemotherapy (HIIC) using a self retaining retractor. In: Sugarbaker PH (ed) Peritoneal carcinomatosis: principles of management. Kluwer, Boston, pp 415–421
Eksborg S, Ehrsson H, Lindfors A (1983) Liquid chromatographic determination of mitomycin C in human plasma and urine. J Chromatogr 274:263–270
Tjaden UR, de Bruijn EA, van der Hoeven RA, Jol C, van der Greef J, Lingeman H (1987) Automated analysis of mitomycin C in body fluids by high-performance liquid chromatography with on-line sample pre-treatment. J Chromatogr 420:53–62
Jacquet P, Sugarbaker PH (1996) Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res 15:49–58
Ronnet BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM (1995) Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis: a clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol 19:1390–1408
Stephens AD, Alderman R, Chang D, Edwards GD, Esquivel J, Sebbag G, Steves MA, Sugarbaker PH (1999) Morbidity and mortality of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the Coliseum technique. Ann Surg Oncol 6:790–796
Van Ruth S, Verwaal VJ, Zoetmulder FAN (2003) Pharmacokinetics of intraperitoneal mitomycin C. Surg Oncol Clin N Am 12:771–780
Elias DM, Sideris L (2003) Pharmacokinetics of heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis. Surg Oncol Clin N Am 12:755–769
De Lima Vazquez V, Stuart OA, Sugarbaker PH (2003) Extent of parietal peritonectomy does not change intraperitoneal chemotherapy pharmacokinetics. Cancer Chem Pharmacol 52:108–112
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugarbaker, P.H., Stuart, O.A. & Carmignani, C.P. Pharmacokinetic changes induced by the volume of chemotherapy solution in patients treated with hyperthermic intraperitoneal mitomycin C. Cancer Chemother Pharmacol 57, 703–708 (2006). https://doi.org/10.1007/s00280-005-0074-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-0074-8