Abstract
Thrombosis in myeloproliferative neoplasms (MPNs) is an important clinical problem, and risk-stratified management is essential. To identify the clinical characteristics of thrombosis in patients with MPNs, a nationwide multi-institutional retrospective analysis (JSH-MPN-R18) was conducted. The aim of the present study was to perform a sub-analysis of JSH-MPN-R18 findings to clarify the predictive parameters for thrombosis among complete blood count (CBC) results. Among the patients enrolled in JSH-MPN-R18, those with essential thrombocythemia (ET; n = 1152) and polycythemia vera (PV; n = 456) were investigated. We analyzed and compared CBC parameters between patients with and those without any thrombotic events using Welch’s T-test. Statistical analyses were performed using the R statistical software. Thrombotic events were observed in 74 patients with ET. In multivariate analysis, only the neutrophil ratio was slightly but significantly higher for ET patients with thrombosis than for those without (p < 0.05). Of note, the absolute neutrophil count (aNeu) was considered a useful predictive tool for thrombosis among patients classified as low-risk according to the revised International Prognostic Score of Thrombosis for Essential Thrombocythemia. Among PV patients, those with thrombosis showed significantly higher hematocrit and aNeu than did those without thrombosis. As a thrombosis-associated factor, the neutrophil ratio was slightly but significantly elevated in patients with ET. This myeloid skew might reflect a higher value of JAK2 V617F allelic frequency in patients with ET with thrombosis; this was not clarified in JSH-MPN-R18. Further accumulation of evidence, including genetic information for JAK2 and other passenger mutations, is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential thrombocythemia (ET) and polycythemia vera (PV), classified under Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs), represent unique clinical entities with excessive production of platelets and/or erythroid cells [1]. Despite their generally benign nature, both conditions carry a predisposition for thrombotic events, which influence patient prognosis. Within the framework of MPN management, stratified approaches to thrombosis prevention are instrumental and underscore the need for robust risk assessment tools.
While international criteria such as the European LeukemiaNet (ELN) criteria [2], International Prognostic Score of Thrombosis for Essential Thrombocythemia (IPSET-thrombosis) [3], and revised-IPSET [4] have been instrumental in risk stratification for ET, the ELN criteria [5] and the criteria proposed by Tefferi et al. [6] have been useful for PV, and their application has been predominantly validated in Western populations. The JSH-MPN-R18 trial, a nationwide retrospective analysis conducted in Japan, extended these findings to Asian populations and revealed potential cross-racial applicability [7, 8].
In the evolving landscape of thrombotic risk assessment for MPNs, leukocytosis [9,10,11,12,13,14,15] and neutrophil-to-lymphocyte ratio (NLR) [16, 17] have emerged as promising markers of thrombotic events and mortality. However, extrapolation of these findings to a Japanese cohort remains unexplored. This study aimed to bridge this knowledge gap by leveraging data from the JSH-MPN-R18 trial to ascertain the prognostic value of the neutrophil ratio in thrombosis prediction within a Japanese MPN population.
Methods
Study design
This multicenter retrospective analysis was performed under the auspices of the Japanese Society of Hematology (JSH), to examine the clinical profiles of Japanese patients diagnosed with PV or ET. Participants were part of the JSH-MPN-R18 cohort, aged 20 years or older, and received a diagnosis of PV or ET according to the WHO 2008 or 2017 criteria [1, 18] during the period from April 2005 to March 2018. Details of the inclusion and exclusion criteria have been described previously [7]. This retrospective analysis involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethical approval was granted by the Mie University Hospital Ethical Committee (approval number H2022-165).
Data collection
Diagnostic laboratory data of the enrolled patients were collected retrospectively. Medical records included date of diagnosis, age at diagnosis, sex, presence of driver gene mutations, laboratory parameters (white blood cell [WBC] counts, neutrophil ratio [rNeu] to WBC, absolute number of neutrophils [aNeu], red blood cell [RBC] counts, haemoglobin [Hb], haematocrit [Hct], and platelet counts), incidence of post-diagnostic thrombosis, and mortality.
Outcome measures
The primary outcome measure was overall incidence of thrombotic events. Thrombosis-free survival was calculated from the time of diagnosis to the first event of each type. Patients without adverse events were censored at the last follow-up visit.
Statistical analysis
Univariable and multivariable analyses using Cox proportional hazards regression models were conducted to identify risk factors for thrombotic events. Based on previous studies [2,3,4,5,6,7,8], we included WBC, rNeu, RBC, Hb, Hct, and platelet counts in the models. Because of the high collinearity resulting from the similarity between rNeu and aNeu, a multivariate analysis was conducted using rNeu, excluding aNeu. Subjects with missing data for the variables included in the models were excluded from the analysis.
Fine–Gray competing risk model [19] was used to calculate cumulative incidences of thrombosis. All deaths were considered for the cumulative incidence in Fine–Gray analysis. Identified predictors for thrombosis were tested using receiver operating characteristic (ROC) curve analysis, and area under ROC curve values were determined along with their 95% confidence intervals. Decision tree analysis was performed using R package (rpart).
All analyses were two-sided, with a p-value of < 0.05 considered statistically significant. Statistical analyses were conducted using R software (version 4.2).
Results
In this nationwide retrospective analysis of JSH-MPN-R18 results, we included 1152 patients with ET (Table 1) and 596 patients with PV (Online Resource 1). Regarding PV diagnosis, the current cohort included data from before JAK2 mutation tests became available in Japan, and some cases lacked information on the JAK2 mutation, as observed in raw data of PV cases (Online Resource 1). Given the high number of patients who did not harbour JAK2 mutations, we excluded cases where the JAK2 mutation was negative or unknown from further analysis. Finally, a total of 456 cases with PV were evaluated in the following study, including 446 and 10 patients with JAK2 V617F and JAK2 exon12 mutation, respectively.
Table 1 outlines the baseline characteristics, laboratory data, and mutation status. Among the patients, 74 and 22 cases of ET and PV, respectively, developed thrombosis, as shown in Table 2. To evaluate the utility of clinical items against future thrombosis, we performed a multivariate analysis. For patients with ET, rNeu was a significant factor associated with thrombosis (Table 3). Besides, decision tree analysis identified rNeu as the most important factor for future thrombosis (see figure, Online Resource 1), and hematocrit was found to be a significant predictive factor for PV (data not shown) in decision tree analysis.
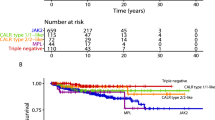
For patients with ET, we defined the rNeu cut-off value as 80% (predicted by decision tree analysis; see figure, Online Resource 2). Fine–Gray cumulative incidence analysis for patients stratified by rNeu revealed that those with high rNeu were more susceptible to future thrombosis (Fig. 1A). We also analyzed the role of aNeu in thrombosis, and the ROC analysis identified an aNeu cutoff value of 5.0 × 109 /L and revealed the potential role of aNeu in thrombosis (Fig. 1B). Interestingly, the importance of aNeu was emphasized in the lower thrombotic risk group (including low/very low in revised-IPSET) (Fig. 2).
Thrombosis-free survival using Fine–Gray analysis The cumulative incidence of thrombosis from the time of essential thrombocythemia (ET) diagnosis was calculated for patients with neutrophilia and those without neutrophilia. The definition of neutrophilia was based on the neutrophil ratio (cut-off value, 80%) and absolute neutrophil count (cut-off value, 5000/µL). The cumulative incidence was significantly lower (Fine–Gray test, p = 0.0152 and p = 0.00073, respectively) for ET patients with neutrophilia than for those without neutrophilia
Thrombosis-free survival according to the revised International Prognostic Score of Thrombosis for Essential Thrombocythemia (rIPSET). The cumulative incidence of thrombosis was calculated for patients with essential thrombocythemia (ET) with high rIPSET and those with low rIPSET. Fine–Gray analysis revealed the significance of aNEU for ET patients with low rIPSET, but not for those with high rIPSET
As there was a significantly higher incidence of patients with JAK2 mutations (Chi-square test, p-value = 5.22 × 10− 14) in the group with rNeu values of ≥ 80%, we further investigated whether the predictive value of rNeu could be applied to all driver mutations. The multivariate analysis identified rNeu as a predictive tool for thrombosis only in JAK2 mutated ETs but not in CALR/MPL mutated ETs (Table 3).
Next, we performed a similar analysis in patients with PV. The multivariate analysis revealed that Hct was a predictive factor for thrombosis, as predicted. Previous reports [12, 20] have also highlighted that a higher WBC count can be a predictive tool for thrombosis; however, there has been little evidence of the predictive value of aNeu and rNeu for thrombosis in our dataset. ROC analysis of WBC against thrombosis revealed a cut-off value of 7.05 × 109/L (Fig. 3A). The Fine–Gray method revealed that thrombosis rates for JAK2-positive PV with aNeu ≥ 7.05 × 109/L were higher, although the difference was not statistically significant (Fig. 3B).
Prognostic utility of the absolute neutrophil count for thrombosis
Receiver operating characteristic (ROC) curve analysis was performed for the neutrophil ratio (rNeu) against future thrombosis for patients with polycythemia vera (PV) (A). The cumulative incidence of thrombosis from the time of PV diagnosis in patients with neutrophilia (aNeu ≥ 7000) and those with lower neutrophil counts was calculated (B). The incidence of thrombosis was significantly lower (Fine–Gray test, p = 0.0295) for patients with PV and neutrophilia than for those without neutrophilia
Discussion
This analysis of the JSH-MPN-R18 dataset underscores the prognostic significance of elevated neutrophil levels in predicting thrombotic events in patients with MPNs. Similar to previous studies that emphasized the NLR as a risk marker, our study revealed that increased rNeu and aNeu were associated with a greater risk of thrombosis.
The biological mechanisms linking heightened neutrophil activity to thrombotic complications remain unclear. However, the observed correlation was particularly strong in ET patients with JAK2 mutations; this implied that JAK2 mutations are associated with thrombosis. Previous studies have identified the ERK pathway as JAK2-specific [21] and characteristic of neutrophil activation [22, 23]. Furthermore, as JAK2 V617F allelic burden in MPNs correlates with leukocyte counts, higher rNeu might reflect higher JAK2 signaling activity [23, 24].
Our study has certain limitations, notably the absence of lymphocyte counts, which prevents a complete evaluation of the predictive value of NLR or rNeu for thrombosis. In addition, we did not have molecular background or allelic frequency information of the driver mutations. Registration of patients with MPNs began in 2005, and continuous follow-up for therapeutic interventions was not included. Because of incomplete data for some cases, we could not uniformly apply the latest WHO classification for disease definition. These limitations indicate that our study is not comprehensive, and future validation is warranted. To overcome these limitations, we are planning a novel cohort study in Japan. Nonetheless, our data showed that simple rNeu can identify ET patients with a high risk of thrombosis. Further data collection on these risk factors is required in future studies.
In conclusion, our study underscores the significance of rNeu, aNeu, and JAK2 mutations in predicting thrombosis in Japanese patients with ET. Further accumulation of evidence and the development of new therapeutic strategies tailored to the unique characteristics of Japanese patients are imperative. Preventive interventions that consider the specific genetic and clinical traits of Japanese patients may play a key role in mitigating the thrombotic risks associated with MPNs.
Data availability
The data that support the findings of this study are available on request from the corresponding author, KN.
Change history
21 August 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00277-024-05940-4
References
Swerdlow SH et al (2017) WHO classification of tumours of haematopoietic and lymphoid tissues, rev, 4th edn. IARC, Lyon, France
Barbui T et al (2018) Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia 32(5):1057–1069. https://doi.org/10.1038/s41375-018-0077-1
Barbui T et al (2012) Development and validation of an International Prognostic score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood 120(26):5128–5133 quiz 5252. https://doi.org/10.1182/blood-2012-07-444067
Barbui T et al (2015) Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 5(11):e369. https://doi.org/10.1038/bcj.2015.94
Barbui T et al (2011) Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 29(6):761–770. https://doi.org/10.1200/JCO.2010.31.8436
Tefferi A, Spivak JL (2005) Polycythemia vera: scientific advances and current practice. Semin Hematol 42(4):206–220. https://doi.org/10.1053/j.seminhematol.2005.08.003
Edahiro Y et al (2022) Clinical characteristics of Japanese patients with polycythemia vera: results of the JSH-MPN-R18 study. Int J Hematol 116(5):696–711. https://doi.org/10.1007/s12185-022-03412-x
Hashimoto Y et al (2022) Clinical characteristics, prognostic factors, and outcomes of patients with essential thrombocythemia in Japan: the JSH-MPN-R18 study. Int J Hematol 115(2):208–221. https://doi.org/10.1007/s12185-021-03253-0
Bonicelli G et al (2013) Leucocytosis and thrombosis at diagnosis are associated with poor survival in polycythaemia vera: a population-based study of 327 patients. Br J Haematol 160(2):251–254. https://doi.org/10.1111/bjh.12117
Tefferi A et al (2013) Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 27(9):1874–1881. https://doi.org/10.1038/leu.2013.163
Carobbio A et al (2019) Leukocytosis and thrombosis in essential thrombocythemia and polycythemia vera: a systematic review and meta-analysis. Blood Adv 3(11):1729–1737. https://doi.org/10.1182/bloodadvances.2019000211
Krecak I et al (2024) The triple A model (age, absolute neutrophil count, absolute lymphocyte count-AAA) predicts survival and thrombosis in polycythemia vera. Am J Hematol 99(5):989–992. https://doi.org/10.1002/ajh.27261
Lucijanic M et al (2024) Evaluation of absolute neutrophil, lymphocyte and platelet count and their ratios as predictors of thrombotic risk in patients with prefibrotic and overt myelofibrosis. Life (Basel) 14(4):523. https://doi.org/10.3390/life14040523
Barbui T et al (2009) Perspectives on thrombosis in essential thrombocythemia and polycythemia vera: is leukocytosis a causative factor? Blood 114(4):759–763. https://doi.org/10.1182/blood-2009-02-206797
Falanga A et al (2005) Pathogenesis of thrombosis in essential thrombocythemia and polycythemia Vera: the role of neutrophils. Semin Hematol 42(4):239–247. https://doi.org/10.1053/j.seminhematol.2005.05.023
Carobbio A et al (2022) Neutrophil-to-lymphocyte ratio is a novel predictor of venous thrombosis in polycythemia vera. Blood Cancer J 12(2):28. https://doi.org/10.1038/s41408-022-00625-5
Kwon SS et al (2022) Neutrophil-lymphocyte ratio and carotid plaque burden in patients with essential thrombocythemia and polycythemia vera. Nutr Metab Cardiovasc Dis 32(8):1913–1916. https://doi.org/10.1016/j.numecd.2022.04.013
Swerdlow SH et al (2008) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC, Lyon, France
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94(446):496–509. https://doi.org/10.1080/01621459.1999.10474144
Barbui T et al (2015) White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood 126(4):560–561. https://doi.org/10.1182/blood-2015-04-638593
Brkic S et al (2021) Dual targeting of JAK2 and ERK interferes with the myeloproliferative neoplasm clone and enhances therapeutic efficacy. Leukemia 35(10):2875–2884. https://doi.org/10.1038/s41375-021-01391-2
Oku S et al (2010) JAK2 V617F uses distinct signalling pathways to induce cell proliferation and neutrophil activation. Br J Haematol 150(3):334–344. https://doi.org/10.1111/j.1365-2141.2010.08249.x
Vannucchi AM et al (2007) Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia 21(9):1952–1959. https://doi.org/10.1038/sj.leu.2404854
Moliterno AR, Kaizer H, Reeves BN (2023) JAK2 V617F allele burden in polycythemia vera: burden of proof. Blood 141(16):1934–1942. https://doi.org/10.1182/blood.2022017697
Acknowledgements
We thank all contributors of the JSH-MPN-R18 trial.We thank Editage for editing and reviewing the manuscript for English language.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
KN performed statistical analysis and wrote the paper. EO, YE, YH, TI, AG, MN, FK, MK, KK, HW, KU, TT, TM, SW, TS, AS, KS, TK, AT, HK, KA, IM, NK, KO and IT collected data and helped the paper writing. KT and YS supervised the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethical approval for this study was granted by the Mie University Hospital Ethical Committee (approval number H2022-165).
Consent to participate
This study is retrospective in nature; therefore, the requirement for informed consent was waived by the ethics committee.
Consent for publication
Not applicable.
Competing interests
Keiki Nagaharu reports research funds under Takeda Pharmaceutical internationals Yoko Edahiro reports research funds under contract from Meiji Seika Pharma, and an endowed chair funded by PharmaEssentia Japan K.K. Yoshinori Hashimoto reports honoraria from Takeda Pharmaceutical and Novartis Pharmaceutical, and an endowed chair funded by PharmaEssentia Japan K.K. Tomoki Ito reports honoraria from Novartis Pharmaceutical, Sanofi, and Takeda Pharmaceutical, and honoraria and grants from Abbvie Inc. and Bristol-Meyer Squib. Akihiko Gotoh reports honoraria from Novartis Pharma K.K., Alexion Pharmaceuticals, Inc., Eisai Co., Ltd., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Nihon Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Janssen Pharmaceutical K.K., Pfizer Japan Inc., Sanofi K.K., PharmaEssentia Japan K.K. Mika Nakamae reports research fund from Veritas Corporation, honoraria to her spouse from Amgen Inc., Astellas Pharma, AstraZeneca plc., Bristol-Meyer Squib, Daiichi Sankyo, Janssen Pharmaceutical K.K., Meiji Seika Pharma Co., Ltd., Nippon Shinyaku, Novartis Pharmaceutical, Ono Pharmaceutical, Otsuka Pharmaceutical, Sumitomo Pharma, and Takeda Pharmaceutical. Fumihiko Kimura reports grants from Ono Pharmaceutical and Takeda Pharmaceutical. Keita Kirito reports honoraria from Novartis Pharmaceutical, Takeda Pharmaceutical and PharmaEssentia JAPAN. Hideho Wada reports grants from Chugai Pharmaceutical, Kyowa Kirin, Ono Pharmaceutical, and Takeda Pharmaceutical. Kensuke Usuki reports research funds under contract from Abbvie Inc., Amgen Astellas BioPharma, Apellis Pharmaceutical, Astellas Pharma, Bristol-Meyer Squib, Daiichi Sankyo, Janssen Pharmaceutical, Nippon Shinyaku Pharmaceutical, Otsuka Pharmaceutical, SymBio Pharmaceutical, and Takeda Pharmaceutical, and honoraria and research funds under contract from Novartis Pharmaceutical. Takehiko Mori reports honoraria from Pfizer, Novartis Pharmaceutical, Takeda Pharmaceutical, PharmaEssentia Japan, and research funds under contract from Asahi Kasei Pharma, Chugai Pharmaceutical, Kyowa Kirin, Eisai Pharmaceutical, JCR Pharmaceuticals, Abbvie Inc., Sumitomo Pharma, CSL Behring, Japan Blood Products Organization and Otsuka Pharmaceutical. Kazuya Shimoda reports honoraria from Bristol-Meyer Squib, Celgene, Novartis Pharmaceutical, and Takeda Pharmaceutical, research funds under contract from PharmaEssentia Japan K.K., and grants from Abbvie Inc., Astellas Pharma, Chugai Pharmaceutical, Kyowa Kirin, and MSD. Akihiro Tomita reports honoraria from Takeda Pharmaceutical; research funds under contract from Kyowa Kirin, Novartis Pharmaceutical, Ono Pharmaceutical, Perseus Proteomics Inc., Pfizer, and Taiho Pharmaceutical; and honoraria and research funds under contract from Chugai Pharmaceutical. Koichi Akashi reports honoraria from Celgene and Novartis Pharmaceutical, grants from Asahi Kasei Pharma, Mochida, MSD, Mundi Pharma, Nippon Shinyaku Pharmaceutical, Ono Pharmaceutical, Shionogi, Sanofi, Sumitomo Dainippon Pharma, Taiho Pharmaceutical, Toyama Chemical, Shin Nippon Biomedical Laboratories, Yakult Honsha, and Takeda Pharmaceutical, honoraria and research funds under contract from Bristol-Meyer Squib and Janssen Pharmaceutical, honoraria and grants from Abbvie Inc., Chugai Pharmaceutical, and Eisai Pharmaceutical, research funds under contract and grants from Daiichi Sankyo and Otsuka Pharmaceutical, honoraria, research funds under contract, and grants from Astellas Pharma and Kyowa Kirin. Itaru Matsumura received speakers bureau fees from Pfizer Japan Inc., Ono Pharmaceutical Co., Ltd., Novartis Pharma K.K., Chugai Pharmaceutical Co., Ltd., AbbVie G.K., Janssen Pharmaceutical K.K., Bristol Myers Squibb K.K. (Celgene K.K.), AstraZeneca K.K., and Otsuka Pharmaceutical Co., Ltd.; received research funding from Chugai Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Sumitomo Pharma Co., Ltd., Nippon Shinyaku Co., Ltd., Eisai Co., Ltd.,Asahi Kasei Pharma Corp, AbbVie G.K., Taiho Pharmaceutical Co., Ltd., and received consultancy fees from Otsuka Pharmaceutical Co., Ltd.Katsuto Takenaka reports honoraria from Novartis Pharmaceutical and MSD, grants from Astellas Pharma, Chugai Pharmaceutical, and Otsuka Pharmaceutical, honoraria, and grants from Kyowa Kirin. Norio Komatsu reports honoraria from Abbvie Inc., Celgene, and Japan Tobacco Inc., research funds under contract from FUJIFILM Wako Chemicals, Fuso-pharm, Meiji Seika Pharma, Perseus Proteomics Inc., and Pfizer, grants from Bristol-Meyer Squib, Chugai Pharmaceutical, Kyowa Kirin, and Sumitomo Dainippon Pharma, honoraria and grants from Novartis Pharmaceutical and Otsuka Pharmaceutical, honoraria, research funds under contract, and grants from Takeda Pharmaceutical, honoraria, research funds under contract, and an endowed chair funded by PharmaEssentia Japan K.K. The other authors declare no conflicts of interest. Kohishi Ohishi reports research funds under contract from PharmaEssentia Japan K.K., Abbvie Inc. Isao Tawara reports honoraria from Novartis Pharmaceutical, Sanofi, and Takeda Pharmaceutical, and honoraria and grants from Abbvie Inc. and Bristol-Meyer Squib.Yuka Sugimoto reports honoraria from PharmaEssentia Japan K.K. and Novartis Pharmaceutical and research funds under contract from Incyte Biosciences, Japan and Toyo Kohan K.K.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: This article was originally published with an outdated ESM files.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagaharu, K., Ohya, E., Edahiro, Y. et al. Predictive significance of high neutrophil ratio for thrombosis in myeloproliferative neoplasms: JSH-MPN-R18 subanalysis. Ann Hematol 103, 3535–3541 (2024). https://doi.org/10.1007/s00277-024-05898-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-024-05898-3