Abstract
The low-dose anti-thymocyte globulin (ATG) plus low-dose post transplantation cyclophosphamide (PTCy) -based (low-dose ATG/PTCy-based) regimen had a promising activity in preventing of graft-versus-host disease (GVHD) in adult patients. However, its efficacy in pediatric patients remain to be defined. Here, we presented the findings from 35 pediatric patients undergoing haploidentical peripheral blood stem cell transplantation (haplo-PBSCT) with the new regimen for GVHD prophylaxis. The cumulative incidences (CIs) of grades II-III and III-IV acute GVHD (aGVHD) were 34% (95% CI, 17–48%) and 11% (95% CI, 0–21%) within 180 days post-transplantation, respectively. The CIs of chronic GVHD (cGVHD) and moderate-to-severe cGVHD within 2 years were 26% (95% CI, 7–41%) and 12% (95% CI, 0–25%), respectively. The 2-year probabilities of overall survival, relapse-free survival, and graft-versus-host disease and relapse-free survival were 89% (95% CI, 78–100%), 82% (95% CI, 68–98%) and 59% (95% CI, 43–80%), respectively. The CIs of cytomegalovirus (CMV) and Epstein-Barr virus (EBV) reactivation by day 180 were 37% (95% CI, 19–51%) and 20% (95% CI, 6–32%) respectively. These results strongly advocate for the efficacy of the low-dose ATG/PTCy-based regimen as a robust strategy for GVHD prevention in haplo-PBSCT for pediatric patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute leukemia constitutes approximately 30% of all childhood malignancies, representing the most prevalent cancer among children [1]. Allogeneic hematopoietic stem cell transplantation (allo-HSCT), as a potentially curative modality for malignant hematologic disorders, significantly improves the survival rates in this demographic [2]. While allo-HSCT from HLA-matched sibling donors (MSD) yields optimal outcomes, the scarcity of such donors due to familial constraints poses a challenge [1, 3]. HLA-haploidentical HSCT (haplo-HSCT) addresses donor shortage and potentially augments graft-versus-leukemia (GVL) effect [4]. Nonetheless, the heightened incidence of graft-versus-host disease (GVHD) associated with haplo-HSCT remains a primary concern, detrimentally impacting overall patient survival [5, 6]. The pathophysiology of acute GVHD (aGVHD) unfolds in three phases: initiation, T cell activation, and effector phases, with T cell activation assuming a central role [7, 8]. Consequently, pharmacological prophylaxis for aGVHD focuses on inhibiting T cell activation and depleting T cells. Cyclosporine A (CsA) or tacrolimus combined with methotrexate or mycophenolate mofetil (MMF) are commonly employed for inhibiting T cell activation [9,10,11], while anti-thymocyte globulin (ATG) and post-transplant cyclophosphamide (PTCy) are prescribed for in vivo T-cell depletion for GVHD prophylaxis for patients undergoing haplo-HSCT [12,13,14,15,16]. The addition of low-dose ATG to PTCy shows promising activity in haploidentical donor settings, demonstrating superior results compared to PTCy or ATG as monotherapy [17, 18]. A novel regimen comprising low-dose ATG (5 mg/kg) and PTCy (50 mg/kg) combined with calcineurin inhibitors (CNIs) and MMF (termed as low dose ATG/PTCy-based regimen) yielded promising outcomes for GVHD prophylaxis in adult haploidentical peripheral blood stem cell transplantation (haplo-PBSCT) [19,20,21,22]. Nonetheless, its applicability in pediatric patients with hematologic malignancies undergoing haplo-PBSCT remains uncertain. Here, we reported the outcomes from 35 pediatric patients undergoing haplo-PBSCT with the low-dose ATG/PTCy-based regimen for GVHD prevention, which suggested that the regimen had a promise activity for GVHD prophylaxis in children.
Patients and methods
Patients
From January 2017 to June 2023, all pediatric patients undergoing haplo-PBSCT in our center received the low dose ATG/PTCy-based regimen for GVHD prophylaxis and were enrolled into the retrospective study. The study had ethical approval from hospital ethical committees and was conducted in accordance with the Declaration of Helsinki. All patients included in the study signed informed consent by their legal guardians.
Donor selection and stem cell source
Family members were chosen as donors based on typing for HLA-A, -B, -C, -DRB1, and -DQB1 loci at a high-resolution level. A haplotype was defined by a recipient-donor pair with ≥ 3 HLA mismatched loci [23]. The graft was from mobilized peripheral blood stem cells (PBSCs) with granulocyte colony stimulating factor (G-CSF) for 5 days at a dose of 10 ug/kg/d. The desired threshold for CD34+ cells in grafts is a minimum of 8 × 106/kg recipient weight.
Conditioning regimens and GVHD prophylaxis
Myeloablative conditioning regimens (MACs) were uniformly employed for all patients in our study. Patients diagnosed with myeloid malignancies received a conditioning regimen consisting of intravenous busulfan (Bu), administered at different dosages based on weight: 0.8 mg/kg/dose for individuals weighing over 34 kg, totaling 16 doses; 0.95 mg/kg/dose for those weighing between 23 and 34 kg, also totaling 16 doses; and 1.1 mg/kg/dose for patients weighing between 16 and 23 kg, totaling 16 doses. Additionally, they received fludarabine at a dose of 30 mg/m²/day and cytarabine (Ara-C) at a dosage of 1–2 g/m²/day for 5 consecutive days. The MAC regimens for lymphoid malignancies comprised 10 Gy fractionated TBI (FTBI), cyclophosphamide (Cy) administered at a dosage of 50 mg/kg/day for 2 days, and etoposide (VP-16) at a dosage of 10 mg/kg/day for 2 days.
GVHD prophylaxis involved the administration of ATG at a dose of 2.5 mg/kg on days − 2 to -1, followed by PTCy at 50 mg/kg on day + 3, and initiation of CsA and MMF on day + 4. CsA was administered as a continuous infusion at a dose of 2 mg/kg/d to achieve nadir serum levels between 200 and 300 ng/ml. MMF was given orally at a dose of 15 mg/kg three times daily (maximum dose of 3 g per day) until day + 34, with discontinuation thereafter in the absence of aGVHD. Mycophenolate sodium enteric-coated tablets (MPA) could be used as an alternative to MMF, with one tablet of MPA being equivalent to one tablet of MMF. CsA was tapered from day + 90 to day + 180 [19,20,21,22].
Supportive care
G-CSF was administered to all patients from day + 5 until neutrophil recovery. Prophylactic levofloxacin and acyclovir were provided to all patients starting from the initiation of conditioning therapy until hematological reconstitution. Additionally, prophylactic posaconazole was administered from the commencement of conditioning therapy until at least three months post-transplant. Quantitative real-time polymerase chain reaction (PCR) assays for CMV DNA in serum and EBV DNA in whole blood were conducted once or twice weekly. Preemptive therapy with ganciclovir (5 mg/kg, twice daily) was initiated if CMV DNA levels exceeded 1000 copies/ml. Similarly, preemptive therapy with rituximab (a single dose of 375 mg/m2) was initiated if EBV DNA levels increased by a logarithmic scale within one week or exceeded 1 × 105 copies/ml in high-risk patients with EBV reactivation [24].
Definitions
Neutrophil engraftment was defined as achieving an absolute neutrophil count (ANC) of ≥ 0.5 × 109/L for 3 consecutive days post-transplantation without G-CSF. Platelet engraftment was defined as attaining a platelet count of ≥ 20 × 109/L for the first of 7 consecutive days without platelet transfusion [25]. Full donor chimerism was delineated as having ≥ 95% donor T cells in BM samples [26]. Graft failure encompassed either the absence of neutrophil engraftment by day 28 post-transplantation (primary graft failure, PGF), or the loss of donor chimerism subsequent to initial engraftment at any point without concomitant disease relapse (secondary graft failure, SGF) [25]. And aGVHD was diagnosed and graded in accordance with the modified Glucksberg criteria [27], while cGVHD was assessed following the 2014 National Institutes of Health consensus criteria [28]. Morphologic complete remission (CR) was determined as per the criteria outlined by the International Working Group (IWG) and National Comprehensive Cancer Network (NCCN) guidelines; patients not meeting the criteria for morphologic CR were deemed to have active disease [29,30,31]. Measurable residual disease (MRD) denotes the presence of leukemia cells at frequencies below that of routine measurement by morphology or cytogenetics, but can be measured at high sensitivity using molecular assays (RT-qPCR) or multiparameter flow cytometry (MFC) [32, 33].
Statistical analysis
Only patients with successful ANC engraftment were evaluated for aGVHD and cGVHD was evaluated only in patients with a minimum follow-up of 180 days. The CI of relapse was calculated from the date of allo-HSCT until relapse. Relapse was defined as the presence of blasts in the peripheral blood (PB) or BM (> 5%) following CR [34]. Non-relapse mortality (NRM) was defined as death from any cause other than relapse. Overall survival (OS) was calculated from the date of stem cell infusion until death from any cause or the end of follow-up. Relapse-free survival (RFS) represented survival without relapse while maintaining continuous CR. GVHD-free and relapse-free survival (GRFS) signified survival without experiencing grade III-IV aGVHD, severe cGVHD, disease relapse, or death from any cause after haplo-PBSCT [35]. Survival curves were plotted using the Kaplan-Meier method. The statistical analyses were performed using IBM SPSS 17.0 statistical software (IBM, North Harbour, Portsmouth, UK).
Results
Patient characteristics
From January 2017 to June 2023, 35 pediatric patients underwent haplo-PBSCT were enrolled in this study. All the patients were diagnosed with hematological malignancies and acute lymphoblastic leukemia (ALL) accounted for 60%, followed by acute meyloid leukemia (AML) for 29%. The clinical characteristics of all the patients are outlined in Table 1.
Engraftment
The median time of neutrophil engraftment was 11 days (range, 9–17), while the median time of platelet engraftment was 11 days (range, 11–22). The chimerism monitoring results revealed that all the patients exhibited full donor chimerism at 28 days post-transplantation. No PGF were observed, but two patients with AML experienced SGF after infections on day 23 and 44 post-transplant, respectively.
Immune reconstitution
Median lymphocyte counts of peripheral blood after transplantion, stratified by CD3+, CD4+, CD8+, CD19+, and CD56/CD16+, are depicted in Fig. 1. On days + 90 and + 120, median CD3+, CD4+, CD8+, CD19+, and CD56/CD16+ counts were 879 (range, 117–2107) and 805 (range, 164–3788), 124 (range, 9–254) and 119 (range, 28–743), 667 (range, 27–1752) and 669 (range, 85–2566), 9 (range, 0–298) and 49 (range, 3–121), 147 (range, 78–1096) and 200 (range, 97–411) per microliter, respectively. Since 210 days post-transplantation, CD4+ cell counts have nearly reached 200 per microliter.
GVHD and infectious complications
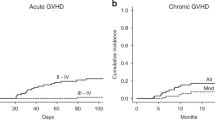
The CIs of grade II-IV and III-IV aGvHD were 34% (95% CI, 17–48%) and 11% (95% CI, 0–21%) within 180 days after transplantation, respectively (Fig. 2a). The CIs of cGVHD and moderate-to-severe cGvHD within 2 years were 26% (95% CI, 7–41%) and 12% (95% CI, 0–25%) respectively (Fig. 2b). The CIs of CMV and EBV reactivation by day + 180 were 37% (95% CI, 19–51%) and 20% (95% CI, 6–32%) respectively. Hemorrhagic cystitis (HC) was diagnosed in four patients with BK virus infection (11%). Ten patients suffered from pneumonia, including 2 patients with bacterial pneumonia, 2 with virus pneumonia, 4 with mixed-pathogen pneumonia, and 2 with unexplained pneumonia. No patient was diagnosed with post-transplant lymphoproliferative disorder (PTLD). Only one patient developed hepatic sinusoidal obstruction syndrome (SOS) and recovered after systemic corticosteroids and defibrotide treatment.
Relapse, NRM and survival
With a median follow-up of 15 months (range, 3–60 months), four cases experienced relapse. The 2-year cumulative incidence of relapse (CIR) was 16% (95% CI, 0–29%) for all patients (Fig. 3a). Out of the four relapsed patients, three died, including one with B-cell acute lymphoblastic leukemia (B-ALL) achieving CR2 with MRD + before transplantation, one with AML in NR status before transplantation, and one with AML achieving CR1 with MRD-. One individual remains alive for 36 months after achieving CR2 from anti-CD19 chimeric antigen receptor T-cell (CAR-T) therapy, who was diagnosed as B-ALL and achieved CR1 with MRD- before transplantation. One patient succumbed to CMV pneumonia at three months post-transplantation (non-relapse mortality, 3%, 95% CI, 0–8%) (Fig. 3b). No patient died of GVHD. The 2-year probabilities of overall survival (OS, Fig. 3c), relapse-free survival (RFS, Fig. 3d), and GVHD- and relapse-free survival (GRFS, Fig. 3e) were 89% (95% CI, 78–100%), 82% (95% CI, 68–98%) and 59% (95% CI, 43–80%), respectively. All the two patients with active disease achieved CR after transplantation, but one patient relapsed at 4 months post-transplant. At the time of transplantation, the two patients had active diseases with blast percentages of 25% and 65%, respectively.
Out of our 35 patients, 33 achieved CR before transplantation, including 17 who were MRD + and 16 who were MRD-. The CIR for MRD- patients was 20% (95% CI, 0–41%), with overall OS of 84% (95% CI, 66–100%); while for MRD + patients, the CIR was 14% (95% CI, 0–31%), with OS of 94% (95% CI, 83–100%). The CIR and OS between MRD + and MRD- patients were similar. (p = 0.9 for CIR; p = 0.5 for OS).
Discussion
Treatment strategies for pediatric hematologic malignancies are often extrapolated from adult protocols, while the differences in biological processes and epigenetic modifications may lead to varying treatment responses [36,37,38]. Hence, despite the success of the low-dose ATG/PTCy-based regimen for adults, evaluating its efficacy in GVHD prophylaxis for children undergoing haplo-PBSCT is necessary. Here, we demonstrated the novel regimen for GVHD prophylaxis in haplo-PBSCT for pediatric hematologic malignancies had a promising efficiency.
All the pediatric patients successfully engrafted after transplantation except for two patients experiencing secondary graft failure. The median time for neutrophil engraftment (11 days) was comparable to that observed in adult patients (12 days) [19]. The reconstitution of neutrophil was faster with the novel regimen as compared with PTCy (20.5 days) and ATG-based (21 days) regimens for GVHD prophylaxis in pediatric patients [39, 40]. In our study, the median CD4+ lymphocyte counts were 124/µl on day + 90, akin to the counts in adults on day + 100 (104/µl). The implementation of our novel protocol indicates that the median lymphocyte counts among pediatric transplant recipients closely mirrors the findings from our prior research conducted with adult subjects [19]. Moreover, it even surpasses the efficacy of the regimen involving tacrolimus, mycophenolate mofetil and cyclophosphamide (50 mg/kg/day on days + 3 and + 4) in children [39]. Our previous studies have demonstrated that the low-dose ATG/PTCy-based regimen did not affect the hematopoietic reconstitution and immune reconstitution time was compared to that in ATG-based regimens for haplo-PBSCT in adults [19].
In the present study, the CIs of grades II-IV and III-IV aGVHD within 180 days post-transplant were 34% (95% CI, 17–48%) and 11% (95% CI, 0–21%), respectively, which were slightly higher than those observed in adult patients in our previous studies [19, 21, 22]. In the initial, PTCy regimen alone was performed in adult patients for GVHD prophylaxis with haploidentitcal bone marrow transplantation (haplo-BMT) and was demonstrated to have a promising efficiency for GVHD prophylaxis [14, 41]. However, when the mobilized peripheral blood stem cells (PBSCs) were utilized as graft substituted for BM cells, the prevention efficiency for GVHD was significantly reduced [42]. The results of GVHD prophylaxis for pediatric patients undergoing haplo-HSCT with PTCy regimen were similar with adults. The frequencies of grade II-IV and III-IV aGVHD for haplo-BMT in pediatric patients were 19–33% and 5-12.5%, respectively [43, 44], while for haplo-PBSCT, were increased with that of 43% and 17%, respectively [45]. In our present study for haplo-PBSCT in pediatric patients, the incidences of grade II-IV and III-IV aGVHD were 34% (95% CI, 17–48%) and 11% (95% CI, 0–21%), respectively, which seems to be lower than that with PTCy alone for GVHD prophylaxis [45]. The reason for the higher incidence of aGVHD in pediatric patients under 18 years old compared to the results observed in adults over 18 years old in our previous research is suspected to be due to the donors being much older than the pediatric patients. Donors for pediatric patients under 18 years old are frequently their significantly older parents, whereas for adult patients over 18 years old, donors typically consist of siblings of similar age or younger offspring. The high incidence of aGVHD among pediatric patients under 18 years old has also been observed in studies conducted at other institutions [46]. However, it is noteworthy that the occurrence of aGVHD in children aged 2–12 is lower compared to both children aged 12–18 and adults over 18 [47]. The precise reasons for this disparity remain unclear. Given that children aged 2–12 constitute a relatively small proportion of our study population (16%), it is plausible that the incidence of aGVHD appears elevated compared to previous studies involving adults. The CIR of overall cGVHD and moderate-to-severe cGVHD within 2 years were 26% (95% CI, 7–41%) and 12% (95% CI, 0–25%), respectively. They were comparable to the incidences observed in adult patients at our center, which were 31% (95% CI, 25-37%) and 18% (95% CI, 14-23%), respectively [22]. This indicates the low-dose ATG/PTCy-based regimen is an effective regimen for preventing GVHD in haplo-PBSCT.
Disease recurrence after allo-HSCT remains a significant concern, particularly when GVHD is effectively controlled, which is especially pertinent for children with higher treatment expectations. In our study, four out of thirty-five cases experienced disease relapse, resulting in three fatalities. This outcome is more ideal than the recurrence rate of 17–30% observed in other schemes [40, 48, 49]. However, due to the shorter follow-up duration and limited sample size in our patient cohort, the impact of our novel GVHD prophylaxis regimen on relapse necessitates longer follow-up and a larger sample size for further evaluation.
During the last two decades, accumulating evidence has shown that pre-transplant MRD status correlates with the risk of relapse and OS after HSCT and patients with positive MRD are risk factors for recurrence [50, 51]. In our study, the CIR and OS between MRD + and MRD- patients were similar (p = 0.9 for CIR; p = 0.5 for OS). This phenomenon may be due to our small sample size, short follow-up period, and significant heterogeneity among patients, including different leukemia subtypes, disease severity, and treatment regimens.
In previous studies, mortality resulting from GVHD and infection constituted the predominant contributors to NRM in allo-HSCT [52]. In our study, the NRM rate stood at 3% among children, a rates significantly lower than the 9% observed in adults. No deaths were attributed to GVHD, while one patient succumbed to pneumonia caused by CMV. Furthermore, the CIs of CMV and EBV reactivation by day + 180 were 37% (95% CI, 19–51%) and 20% (95% CI, 6–32%) respectively, with no instances of PTLD, aligning with rates observed in adults (38% and 41%). Hemorrhagic cystitis associated with BK virus infection was noted in 11% of patients, comparable to the incidence in adult patients [19]. Consistent with findings among adult subjects, children who implemented our preventive regimen exhibited a comparable infection rate to those employing PTCy alone, while was demonstrated to have a superior outcome compared to those using ATG alone. For instance, The CI of CMV reactivation by day + 180 were 37%, which was lower than with ATG-based regimens (55–71%) and comparable to the PTCy-based regimen (35%) [43, 53, 54]. These findings underscore the efficacy of the low-dose ATG/PTCy regimen-based regimen in reducing NRM by effectively preventing GVHD in children, outperforming its impact in adults. As discussed in the preceding text, the immune recovery in our study mirrored the swiftness observed in our previous experiences with adults and is faster than other schemes.
Conclusion
The main limitations of this study were the small cohort of patients, the short follow-up and the absence of a randomized trial. Nevertheless, it is important to highlight that the low-dose ATG/PTCy-based regimen as GVHD prophylaxis in haploidentical PBSCT resulted in a low incidence of GVHD with reasonable outcomes in children with hematologic malignancies. Further studies with high methodological quality, such as larger sample sizes and randomized and controlled trials, are required to compare the efficacies of this regimenin children.
Data availability
The data that support the findings of this study are available on request from the corresponding author, [S.X.],upon reasonable request.
References
Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, Hartzman R, Rizzo JD, Horowitz M, Confer D, Maiers M (2014) HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med 371(4):339–348. https://doi.org/10.1056/NEJMsa1311707
Thol F, Döhner H, Ganser A (2024) How I treat refractory and relapsed acute myeloid leukemia. Blood 143(1):11–20. https://doi.org/10.1182/blood.2023022481
Raj K, Eikema DJ, Sheth V, Koster L, de Wreede LC, Blaise D, Di Grazia C, Koc Y, Potter V, Chevallier P, Lopez-Corral L, Wu D, Mielke S, Maertens J, Meijer E, Huynh A, Passweg J, Luft T, Pérez-Simón JA, Ciceri F, Piekarska A, Hayri Ozsan G, Kröger N, Robin M, Yakoub-Agha I (2022) Comparison of outcomes for HLA-matched sibling and haplo-identical donors in myelodysplastic syndromes: report from the chronic malignancies working party of EBMT. Blood Cancer J 12(9):140. https://doi.org/10.1038/s41408-022-00729-y
Weisdorf D (2018) Can haploidentical transplantation meet all patients’ needs? Best Pract Res Clin Haematol 31(4):410–413. https://doi.org/10.1016/j.beha.2018.09.012
Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ (2001) Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood 98(12):3456–3464. https://doi.org/10.1182/blood.v98.12.3456
Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W, Chen H, Chen YH, Wang FR, Wang JZ, Sun YQ, Huang XJ (2013) Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer 119(5):978–985. https://doi.org/10.1002/cncr.27761
Malard F, Holler E, Sandmaier BM, Huang H, Mohty M (2023) Acute graft-versus-host disease. Nat Rev Dis Primers 9(1):27. https://doi.org/10.1038/s41572-023-00438-1
Hansen JA, Chien JW, Warren EH, Zhao LP, Martin PJ (2010) Defining genetic risk for graft-versus-host disease and mortality following allogeneic hematopoietic stem cell transplantation. Curr Opin Hematol 17(6):483–492. https://doi.org/10.1097/MOH.0b013e32833eb770
Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Doney K, Farewell V et al (1986) Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 314(12):729–735. https://doi.org/10.1056/nejm198603203141201
Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, Bensinger W, Berenson R, Buckner CD, Clift R et al (1989) Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood 73(6):1729–1734
Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E, Kalaycio M (2004) A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transpl 34(7):621–625. https://doi.org/10.1038/sj.bmt.1704647
Mohty M (2007) Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 21(7):1387–1394. https://doi.org/10.1038/sj.leu.2404683
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, Oneto R, Bruno B, Barbanti M, Sacchi N, Van Lint MT, Bosi A (2001) Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 98(10):2942–2947. https://doi.org/10.1182/blood.v98.10.2942
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, Matsui W, Bolaños-Meade J, Borrello I, Powell JD, Harrington E, Warnock S, Flowers M, Brodsky RA, Sandmaier BM, Storb RF, Jones RJ, Fuchs EJ (2008) HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl 14(6):641–650. https://doi.org/10.1016/j.bbmt.2008.03.005
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, Morris LE, Solomon SR (2013) T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol 31(10):1310–1316. https://doi.org/10.1200/jco.2012.44.3523
Wang Y, Wu DP, Liu QF, Xu LP, Liu KY, Zhang XH, Yu WJ, Xu Y, Huang F, Huang XJ (2019) Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol 12(1):88. https://doi.org/10.1186/s13045-019-0781-y
El-Cheikh J, Devillier R, Dulery R, Massoud R, Al Chami F, Ghaoui N, Moukalled N, Pagliardini T, Marino F, Malard F, Bazarbachi AH, Mohty R, Bazarbachi A, Castagna L, Mohty M, Blaise D (2020) Impact of adding Antithymocyte globulin to Posttransplantation Cyclophosphamide in Haploidentical stem-cell transplantation. Clin Lymphoma Myeloma Leuk 20(9):617–623. https://doi.org/10.1016/j.clml.2020.04.003
Wu KH, Weng TF, Li JP, Chao YH (2022) Antithymocyte Globulin Plus Post-transplant Cyclophosphamide Combination as an effective strategy for graft-versus-host Disease Prevention in Haploidentical Peripheral blood stem cell transplantation for children with high-risk malignancies. Pharmaceuticals (Basel) 15(11). https://doi.org/10.3390/ph15111423
Yang J, Jiang J, Cai Y, Li S, Wan L, Zhu J, Liu H, Shao S, Bai H, Wang C, Song X (2019) Low-dose anti-thymocyte globulin plus low-dose posttransplant cyclophosphamide as graft-versus-host disease prophylaxis in haploidentical peripheral blood stem cell transplantation combined with unrelated cord blood for patients with hematologic malignancies: a prospective, phase II study. Bone Marrow Transpl 54(7):1049–1057. https://doi.org/10.1038/s41409-018-0382-3
Sun X, Yang J, Cai Y, Wan L, Huang C, Qiu H, Tong Y, Xu X, Zhou K, Ding X, Song X (2021) Low-dose antithymocyte globulin plus low-dose posttransplant cyclophosphamide combined with cyclosporine and mycophenolate mofetil for prevention of graft-versus-host disease after HLA-matched unrelated donor peripheral blood stem cell transplantation. Bone Marrow Transpl 56(10):2423–2431. https://doi.org/10.1038/s41409-021-01358-y
Li T, He Q, Yang J, Cai Y, Huang C, Xu X, Qiu H, Niu J, Zhou K, Zhang Y, Xia X, Wei Y, Shen C, Ding X, Tong Y, Wan L, Song X (2022) Low-dose Anti-thymocyte Globulin Plus Low-Dose Posttransplant Cyclophosphamide as an effective regimen for Prophylaxis of Graft Versus host Disease after Haploidentical Peripheral blood stem cell transplantation with Maternal/Collateral related donors. Cell Transpl 31:9636897221139103. https://doi.org/10.1177/09636897221139103
Li X, Yang J, Cai Y, Huang C, Xu X, Qiu H, Niu J, Zhou K, Zhang Y, Xia X, Wei Y, Shen C, Tong Y, Dong B, Wan L, Song X (2023) Low-dose anti-thymocyte globulin plus low-dose post-transplant cyclophosphamide-based regimen for prevention of graft-versus-host disease after haploidentical peripheral blood stem cell transplants: a large sample, long-term follow-up retrospective study. Front Immunol 14:1252879. https://doi.org/10.3389/fimmu.2023.1252879
Munchel AT, Kasamon YL, Fuchs EJ (2011) Treatment of hematological malignancies with nonmyeloablative, HLA-haploidentical bone marrow transplantation and high dose, post-transplantation cyclophosphamide. Best Pract Res Clin Haematol 24(3):359–368. https://doi.org/10.1016/j.beha.2011.05.001
van Esser JW, Niesters HG, van der Holt B, Meijer E, Osterhaus AD, Gratama JW, Verdonck LF, Löwenberg B, Cornelissen JJ (2002) Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood 99(12):4364–4369. https://doi.org/10.1182/blood.v99.12.4364
Martinelli G, Trabetti E, Farabegoli P, Testoni N, Bandini G, Motta MR, Vittone A, Terragna C, Pignatti PF, Tura S (1997) Early detection of bone marrow engraftment by amplification of hypervariable DNA regions. Haematologica 82(2):156–160
Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, Chen H, Wang FR, Mo XD, Zhang YY, Huo MR, Zhao XS, Liu YK, Huang KY XJ (2015) Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol 8:84. https://doi.org/10.1186/s13045-015-0182-9
P D, W D, M P, K HG, B P, H J, T ED (1995) 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl 15(6):825–828
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers ME (2015) National Institutes of Health Consensus Development Project on Criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and Staging Working Group report. Biol Blood Marrow Transpl 21(3):389–401e381. https://doi.org/10.1016/j.bbmt.2014.12.001
Brüggemann M, Schrauder A, Raff T, Pfeifer H, Dworzak M, Ottmann OG, Asnafi V, Baruchel A, Bassan R, Benoit Y, Biondi A, Cavé H, Dombret H, Fielding AK, Foà R, Gökbuget N, Goldstone AH, Goulden N, Henze G, Hoelzer D, Janka-Schaub GE, Macintyre EA, Pieters R, Rambaldi A, Ribera JM, Schmiegelow K, Spinelli O, Stary J, von Stackelberg A, Kneba M, Schrappe M, van Dongen JJ (2010) Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18–20 September 2008. Leukemia 24 (3):521–535. https://doi.org/10.1038/leu.2009.268
Younes A, Hilden P, Coiffier B, Hagenbeek A, Salles G, Wilson W, Seymour JF, Kelly K, Gribben J, Pfreunschuh M, Morschhauser F, Schoder H, Zelenetz AD, Rademaker J, Advani R, Valente N, Fortpied C, Witzig TE, Sehn LH, Engert A, Fisher RI, Zinzani PL, Federico M, Hutchings M, Bollard C, Trneny M, Elsayed YA, Tobinai K, Abramson JS, Fowler N, Goy A, Smith M, Ansell S, Kuruvilla J, Dreyling M, Thieblemont C, Little RF, Aurer I, Van Oers MHJ, Takeshita K, Gopal A, Rule S, de Vos S, Kloos I, Kaminski MS, Meignan M, Schwartz LH, Leonard JP, Schuster SJ, Seshan VE (2017) International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol 28(7):1436–1447. https://doi.org/10.1093/annonc/mdx097
Kruse A, Abdel-Azim N, Kim HN, Ruan Y, Phan V, Ogana H, Wang W, Lee R, Gang EJ, Khazal S, Kim YM (2020) Minimal residual disease detection in Acute Lymphoblastic Leukemia. Int J Mol Sci 21(3). https://doi.org/10.3390/ijms21031054
Della Starza I, Chiaretti S, De Propris MS, Elia L, Cavalli M, De Novi LA, Soscia R, Messina M, Vitale A, Guarini A, Foà R (2019) Minimal residual disease in Acute Lymphoblastic Leukemia: technical and clinical advances. Front Oncol 9:726. https://doi.org/10.3389/fonc.2019.00726
Aitken MJL, Ravandi F, Patel KP, Short NJ (2021) Prognostic and therapeutic implications of measurable residual disease in acute myeloid leukemia. J Hematol Oncol 14(1):137. https://doi.org/10.1186/s13045-021-01148-5
Ruggeri A, Labopin M, Bacigalupo A, Gülbas Z, Koc Y, Blaise D, Bruno B, Irrera G, Tischer J, Diez-Martin JL, Castagna L, Ciceri F, Mohty M, Nagler A (2018) Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer 124(7):1428–1437. https://doi.org/10.1002/cncr.31228
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A (2016) Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transpl 51(4):610–611. https://doi.org/10.1038/bmt.2015.305
Chaudhury SS, Morison JK, Gibson BE, Keeshan K (2015) Insights into cell ontogeny, age, and acute myeloid leukemia. Exp Hematol 43(9):745–755. https://doi.org/10.1016/j.exphem.2015.05.008
Kleinschmidt K, Lv M, Yanir A, Palma J, Lang P, Eyrich M (2021) T-Cell-replete Versus ex vivo T-Cell-depleted Haploidentical Haematopoietic Stem Cell Transplantation in Children with Acute Lymphoblastic Leukaemia and other Haematological malignancies. Front Pediatr 9:794541. https://doi.org/10.3389/fped.2021.794541
Xue YJ, Cheng YF, Lu AD, Wang Y, Zuo YX, Yan CH, Wu J, Sun YQ, Suo P, Chen YH, Chen H, Jia YP, Liu KY, Han W, Xu LP, Zhang LP, Huang XJ (2019) Allogeneic hematopoietic stem cell transplantation, especially haploidentical, May Improve Long-Term Survival for High-Risk Pediatric patients with Philadelphia chromosome-positive Acute Lymphoblastic Leukemia in the tyrosine kinase inhibitor era. Biol Blood Marrow Transpl 25(8):1611–1620. https://doi.org/10.1016/j.bbmt.2018.12.007
Luo R, Zhang X, Wang Y, Man Q, Gu W, Tian Z, Wang J (2022) Post-transplant cyclophosphamide for GVHD prophylaxis in pediatrics with chronic active Epstein-Barr virus infection after haplo-HSCT. Orphanet J Rare Dis 17(1):422. https://doi.org/10.1186/s13023-022-02585-2
Arora S, Thakkar D, Upasana K, Yadav A, Rastogi N, Sharma PS, Yadav SP (2024) Incidence, risk factors, characteristics, and Outcome of Chronic Graft Versus host disease in children undergoing Haploidentical Peripheral Blood Stem Cell Transplant with post-transplant cyclophosphamide. J Pediatr Hematol Oncol 46(1):e44–e50. https://doi.org/10.1097/mph.0000000000002786
Luznik L, Bolaños-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, Huff CA, Borrello I, Matsui W, Powell JD, Kasamon Y, Goodman SN, Hess A, Levitsky HI, Ambinder RF, Jones RJ, Fuchs EJ (2010) High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood 115(16):3224–3230. https://doi.org/10.1182/blood-2009-11-251595
Bashey A, Zhang MJ, McCurdy SR, St Martin A, Argall T, Anasetti C, Ciurea SO, Fasan O, Gaballa S, Hamadani M, Munshi P, Al Malki MM, Nakamura R, O’Donnell PV, Perales MA, Raj K, Romee R, Rowley S, Rocha V, Salit RB, Solh M, Soiffer RJ, Fuchs EJ, Eapen M (2017) Mobilized peripheral blood stem cells Versus Unstimulated Bone Marrow as a graft source for T-Cell-replete Haploidentical Donor Transplantation using post-transplant cyclophosphamide. J Clin Oncol 35(26):3002–3009. https://doi.org/10.1200/jco.2017.72.8428
Klein OR, Buddenbaum J, Tucker N, Chen AR, Gamper CJ, Loeb D, Zambidis E, Llosa NJ, Huo JS, Robey N, Holuba MJ, Kasamon YL, McCurdy SR, Ambinder R, Bolaños-Meade J, Luznik L, Fuchs EJ, Jones RJ, Cooke KR, Symons HJ (2017) Nonmyeloablative haploidentical bone marrow transplantation with Post-transplantation Cyclophosphamide for Pediatric and Young Adult patients with high-risk hematologic malignancies. Biol Blood Marrow Transpl 23(2):325–332. https://doi.org/10.1016/j.bbmt.2016.11.016
Llosa NJ, Cooke KR, Chen AR, Gamper CJ, Klein OR, Zambidis ET, Luber B, Rosner G, Siegel N, Holuba MJ, Robey N, Hayashi M, Jones RJ, Fuchs E, Holdhoff M, Loeb DM, Symons HJ (2017) Reduced-intensity haploidentical bone marrow transplantation with post-transplant cyclophosphamide for solid tumors in Pediatric and Young Adult patients. Biol Blood Marrow Transpl 23(12):2127–2136. https://doi.org/10.1016/j.bbmt.2017.08.012
Trujillo ÁM, Karduss AJ, Suarez G, Pérez R, Ruiz G, Cardona A, Ramírez M, Betancur J (2021) Haploidentical hematopoietic stem cell transplantation with Post-transplantation Cyclophosphamide in Children with High-Risk Leukemia using a reduced-intensity conditioning regimen and peripheral blood as the stem cell source. Transpl Cell Ther 27(5):427e421–427e427. https://doi.org/10.1016/j.jtct.2021.02.010
Vignon M, Andreoli A, Dhédin N, Lengliné E, Masson E, Robin M, Granier C, Larghero J, Schlageter MH, de Latour RP, Socié G, Boissel N (2017) Graft-versus-host disease in adolescents and young adults (15–24 Years Old) after allogeneic hematopoietic stem cell transplantation for Acute Leukemia in First Complete Remission. J Adolesc Young Adult Oncol 6(2):299–306. https://doi.org/10.1089/jayao.2016.0060
Qayed M, Wang T, Hemmer MT, Spellman S, Arora M, Couriel D, Alousi A, Pidala J, Abdel-Azim H, Aljurf M, Ayas M, Bitan M, Cairo M, Choi SW, Dandoy C, Delgado D, Gale RP, Hale G, Frangoul H, Kamble RT, Kharfan-Dabaja M, Lehman L, Levine J, MacMillan M, Marks DI, Nishihori T, Olsson RF, Hematti P, Ringden O, Saad A, Satwani P, Savani BN, Schultz KR, Seo S, Shenoy S, Waller EK, Yu L, Horowitz MM, Horan J (2018) Influence of age on Acute and Chronic GVHD in Children undergoing HLA-Identical sibling bone marrow transplantation for Acute Leukemia: implications for Prophylaxis. Biol Blood Marrow Transpl 24(3):521–528. https://doi.org/10.1016/j.bbmt.2017.11.004
Locatelli F, Bernardo ME, Bertaina A, Rognoni C, Comoli P, Rovelli A, Pession A, Fagioli F, Favre C, Lanino E, Giorgiani G, Merli P, Pagliara D, Prete A, Zecca M (2017) Efficacy of two different doses of rabbit anti-T-lymphocyte globulin to prevent graft-versus-host disease in children with haematological malignancies transplanted from an unrelated donor: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 18(8):1126–1136. https://doi.org/10.1016/s1470-2045(17)30417-5
Admiraal R, Nierkens S, Bierings MB, Bredius RGM, van Vliet I, Jiang Y, Lopez-Yurda M, Versluijs AB, Zwaan CM, Lindemans CA, Boelens JJ (2022) Individualised dosing of anti-thymocyte globulin in paediatric unrelated allogeneic haematopoietic stem-cell transplantation (PARACHUTE): a single-arm, phase 2 clinical trial. Lancet Haematol 9(2):e111–e120. https://doi.org/10.1016/s2352-3026(21)00375-6
Wang Q, Liang Z, Ren H, Dong Y, Yin Y, Wang Q, Liu W, Wang B, Han N, Li Y, Li Y (2023) Real-world outcomes and prognostic factors among patients with acute myeloid leukemia treated with allogeneic hematopoietic stem cell transplantation. Ann Hematol 102(11):3061–3074. https://doi.org/10.1007/s00277-023-05429-6
Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, Estey EH, Salter AI, Lansverk E, Chien JW, Gopal AK, Appelbaum FR, Pagel JM (2011) Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol 29(9):1190–1197. https://doi.org/10.1200/jco.2010.31.8121
Atilla E, Atilla PA, Bozdağ SC, Demirer T (2017) A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection 45(4):403–411. https://doi.org/10.1007/s15010-017-1016-1
Atay D, Akcay A, Yenigurbuz FD, Akinci B, Bagirova K, Hasanova S, Ozturk G (2021) Clinical study of graft-versus-host disease prophylaxis in unrelated hematopoietic stem cell transplantation for pediatric nonmalignant diseases with different doses anti-human T-lymphocyte immunoglobulin. Pediatr Transpl 25(8):e14098. https://doi.org/10.1111/petr.14098
Higuchi K, Sawada A, Kondo O, Okada Y, Tsujimoto H, Ioi A, Mayumi A, Shimizu M, Sato M, Goto K, Inoue S, Yasui M, Inoue M (2022) HLA-haploidentical peripheral blood stem cell transplantation following reduced-intensity conditioning with very low-dose antithymocyte globulin for relapsed/refractory acute leukemia in pediatric patients: a single-institution retrospective analysis. Int J Hematol 115(3):406–413. https://doi.org/10.1007/s12185-021-03270-z
Acknowledgements
This research was funded by a three-year development project from Shanghai Shen Kang Hospital Development Center (SHDC2020CR1012B for Xianmin Song); Clinical Research Innovation Plan of Shanghai General Hospital (CTCCR-2018BP03 for Jun Yang).
Author information
Authors and Affiliations
Contributions
This study was conceived and designed by S.X. ; D.Y. analyzed and interpreted the data and wrote the manuscript; Z.Y. , X.X. , C.Y. , W.Y. , H.C. , Y.J. , Q.H. , N.J. , Z.K , X. X. , S.C. , T.Y. , D.B. , W.L. took care of patients in clinical practice. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of the coordinating institution of Shanghai General Hospital (Code:2022KY023).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, Y., Zhang, Y., Xu, X. et al. Low-dose anti-thymocyte globulin plus low-dose posttransplant cyclophosphamide-based regimen for prevention of graft-versus-host disease in haploidentical peripheral blood stem cell transplantation for pediatric patients with hematologic malignancies. Ann Hematol 103, 3765–3774 (2024). https://doi.org/10.1007/s00277-024-05883-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-024-05883-w