Abstract
With advancements in novel therapeutics, it is unclear whether third hematopoietic cell transplantation (HCT3) has a place in the treatment of recurrent hematopoietic malignancies. We evaluated patients with hematologic malignancies who underwent HCT3 between 2000–2020. Nine patients, with a median age of 18 (9—68) years at HCT3 with acute myelogenous leukemia (n = 5), acute lymphoblastic leukemia (n = 2), myelodysplastic syndrome (n = 1), or undifferentiated acute leukemia (n = 1), were identified. The median time between first HCT and HCT3 was 3.9 (0.7—13.6) years. Indication for HCT3 was relapse (n = 8) or graft failure (n = 1) after second HCT. At HCT3, seven of nine patients were in complete remission by flow cytometry. All experienced robust donor engraftment by one month after HCT3 (≥ 90% CD3) while one died at day + 24 of multi-organ failure and was not evaluable for chimerism. In total, eight patients died from relapse (n = 4), non-relapse, (n = 3) or unknown (n = 1) causes at a median of 0.6 (range, 0.1 – 9.9) years after HCT3. After HCT3, estimated overall survival at 6 months, 1 year, and 5 years was 88%, 63%, and 22%, respectively. In this highly selected group, HCT3 provided a treatment option although long-term survival was still dismal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For some patients with high-risk leukemia, treatment consolidation with hematopoietic cell transplantation (HCT) can often lead to long-term cure. While advances have been made in reducing non-relapse mortality (NRM), relapse continues to be a barrier and limits long-term survival after HCT. Multiple options are available to treat relapse after HCT, including withdrawal of immune suppression, donor lymphocyte infusions (DLI), natural killer (NK) cell infusions, chimeric antigen receptor T-cell therapy, and other targeted drugs and immunomodulatory therapies to either augment graft-versus-leukemia effects or directly eradicate disease [1,2,3,4,5,6,7,8]. However, once remission has been achieved, many groups aim to re-attempt disease consolidation with a second HCT (HCT2) [9,10,11]. To that effect, relapse after first HCT (HCT1) is the leading indication for second allogeneic HCT (HCT2) [12]. However, if this relapse occurs within 6 months following HCT1, it is associated with early relapse after HCT2 and overall worse survival compared to transplants in first complete remission [9,10,11]. While HCT2 is a potentially curative treatment, relapse after HCT2 is often considered incurable. Third HCTs (HCT3) have not been prospectively studied, and few retrospective studies have been reported to guide clinical decision making about the role of this treatment [13, 14]. Furthermore, little is known about the utility of HCT3 in this current era of immunotherapy and directed molecular therapy. Here, we report our institution’s experience and outcomes of patients undergoing HCT3.
Materials and methods
Data collection and analysis
Pediatric and adult patients with acute leukemias or myelodysplastic syndrome (MDS) who underwent HCT3 from an allogeneic donor between January 1, 2000 through October 1, 2020 from the Fred Hutchinson Cancer Center (FHCC) were included in this study. Patients who received an autologous graft for HCT1 were included. Two patients who received an autologous graft for HCT2 or HCT3 were excluded. Data was collected retrospectively via chart review within the electronic medical record of each patient. The retrospective study was approved by the FHCC Institutional Review Board.
Definitions
Pediatric patients were defined as those < 18 years old, while adults were ≥ 18 years old. Conditioning intensity was defined as either reduced-intensity (RIC; including non-myeloablative regimens) or myeloablative based on previous definitions [15, 16]. Acute and chronic graft-versus-host-disease (GVHD) were defined as previously described [17, 18]. HCT comorbidity index (HCT-CI) scores were calculated using previously published criteria [19]. Neutrophil recovery was defined as the first of three consecutive days of absolute neutrophil count ≥ 500 cells/microliter. Platelet recovery was defined as the first of seven consecutive days of platelet count ≥ 50 000 without platelet transfusions. Relapse was defined as any evidence of disease detection based on either bone marrow morphology and/or flow cytometry. Non-relapse mortality (NRM) was defined as any death after HCT caused by events not due to relapse.
Statistical analysis
Patient outcomes were captured from the date of HCT until death or end of follow-up. For incomplete dates, the first day of the month was used. Data cut-off date was December 31, 2020. Overall survival (OS) was calculated using Kaplan–Meier method. Probabilities of NRM and relapse rate were calculated using cumulative incidence estimates to accommodate competing risks. Descriptive statistics were summarized, and categorical variables were presented as percentages. Median value and range were used for continuous variables with non-normal distribution.
Results
Of the 3,795 total allogeneic HCTs performed, a total of nine patients who received HCT3 between 2000 – 2020 were identified (pediatric, n = 5 and adult, n = 4). Of note, all patients were reported to be of White, non-Hispanic/Latino background. Most patients had a diagnosis of acute myeloid leukemia (AML; n = 5) or acute lymphoid leukemia (ALL; n = 2). One patient had MDS and one had acute undifferentiated leukemia (Table 1).
First HCT
First transplants frequently occurred at outside centers (n = 6). Median age at HCT1 was 12 (range 5—56) years. Disease status data was only available for seven patients, all of whom were in a complete morphologic remission. A majority (n = 7) of patients received HCT from 10/10 human leukocyte antigen (HLA)-matched related donors and Patient 1 received an autologous HCT. Five patients received bone marrow (BM) grafts, and four patients received peripheral blood stem cell (PBSC) grafts. Most (n = 8) patients received myeloablative conditioning regimens. All but one relapsed after HCT1 at a median of 1.3 (range, 0.2 – 3.3) years. Patient 2 with MDS experienced graft rejection (Fig. 1).
Second HCT
The median time between HCT1 and HCT2 was 1.6 (range, 0.6—3.7) years, with the median time from relapse to HCT2 of 4.6 (range, 1.9—9.6) months. The median age at HCT2 was 16 (range, 7—57) years. Patient 2 with MDS who experienced graft rejection following HCT1 received DLI as an unsuccessful attempt to improve donor engraftment before proceeding to HCT2. Four patients received HCT2 at outside centers. Most patients received HCT (PBSC, n = 5 and marrow, n = 1) from 10/10 HLA-matched related donors (n = 4, two of which were the same donor as HCT1) or 10/10 HLA-matched unrelated donors (n = 2). Two pediatric patients received cord blood transplants that were HLA-matched 5/6 or 6/6. Patient 9 was transplanted outside of our center and received an HLA-haploidentical donor with unknown stem cell source. Four patients (44%) received myeloablative conditioning regimens. Eight patients relapsed after HCT2 at a median of 0.97 years (range, 0.39 – 11.78; Fig. 1), while Patient 3 with AML experienced de novo graft failure.
Third HCT
All patients received HCT3 at our center. Median time between HCT2 and HCT3 was 1.9 (range, 0.1 – 12.6) years, with median time from relapse to HCT3 of 6.8 (range, 3.8 – 20.6) months. The median age at HCT3 was 18 (range, 9 – 68) years. Details regarding HCT3 characteristics are described in Table 1. Patient 3 underwent HCT3 with the same 10/10 HLA-matched related donor as used in HCT2. Three patients received HLA-haploidentical transplantation, and all three received post-HCT infusions of donor-derived natural killer (NK) cells on a clinical trial. All cord blood recipients (n = 3) underwent myeloablative conditioning while all other patients (n = 6) received RIC conditioning (Table 1). Five patients had HCT-CI scores of ≥ 3, and two patients had HCT-CI scores of 2, while two patients had HCT-CI scores of 0. For the seven patients who had comorbidities contributing to HCT-CI scores, all had moderate (n n = 6) or severe (n = 1) pulmonary involvement. One patient had moderate to severe hepatic and psychiatric comorbidities. Two patients had an infection preceding transplant, and one patient had cardiac dysfunction. Seven of the patients were in complete remission by flow cytometry at time of HCT3. Patient 6 had refractory AML and was transplanted after clearance of peripheral blasts (marrow was not checked), and Patient 7 had 0.07% residual marrow disease at time of HCT3.
Engraftment after HCT3
All patients (n = 9) achieved neutrophil engraftment at a median of 16 (range, 13−48) days following HCT3. Eight patients were evaluable for donor chimerism at one month and all achieved CD3 engraftment (≥ 90%). Seven patients achieved platelet engraftment at a median of 17 (range, 10 – 33) days following HCT3. Two patients did not achieve platelet engraftment before death. In those evaluable, there were no primary or secondary graft rejections or failures after HCT3.
GVHD after HCT3
Following HCT3, eight patients were evaluable for the development of acute and chronic GVHD. Six patients had a maximum acute GVHD grade of II, and one patient had maximum acute GVHD score of III. This latter patient received an HLA-haploidentical marrow graft. For chronic GVHD, one patient developed limited chronic GVHD and had received a 10/10 HLA-matched related PBSC graft. One patient developed extensive chronic GVHD and had received a 9/10 HLA-mismatched unrelated PBSC graft.
Relapse and survival outcomes after HCT3
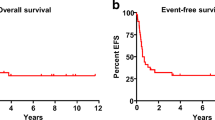
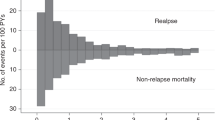
Five patients relapsed following HCT3. Median time to relapse after HCT3 was 5.2 (range, 1.8−5.9) months. After HCT3, estimated relapse rates at 6 months, 1 year, and 5 years post-HCT3 were 63% (Fig. 2a). Estimated overall survival at 6 months, 1 year, 2 years, and 5 years post-HCT3 was 88%, 63%, 33%, and 22% respectively (Fig. 2b). Overall, eight patients died at a median time of 0.6 (range, 0.1−2.9) years after HCT3. Four deaths were due to relapse, three were due to NRM, and one was unknown (Table 2). NRM causes of death included veno-occlusive disease with intraperitoneal and intrapleural hemorrhages, multi-system organ failure (acute lung injury, renal failure, and septic shock), and chronic GVHD. Out of the eight deaths, two (Patients 3 and 6) occurred early at 0.1- and 0.5-years after HCT3 and occurred in patients with HCT-CI scores of 2 and 3, respectively. The three patients with the longest overall survival (Patients 1, 2, and 4) had scores of 0, 0, and 2, with moderate/severe lung disease contributing to the HCT-CI score of 2. Patient 1 survived until 9.9 years after HCT3 and died of unknown causes, and Patient 4 was treated for post-HCT relapse and is currently alive at 7.5 years following HCT3 (Table 2).
Discussion
This study aimed to explore the characteristics of patients who underwent HCT3 and their outcomes at a single center. Our experience suggests that HCT3 is rarely used (0.2% of HCTs conducted at FHCC during the time period studied) but it is possible to have long-term survival in rare cases. Three patients were alive at two years, and of these three, two patients remained alive at five years after HCT3. Factors previously reported to be associated with improved OS after HCT3 included high performance status, robust remission status at time of transplant, and longer length of time from HCT2 to relapse [13, 14]. In our small study, we had a single long-term survivor who was able to maintain durable remission after CAR-T therapy.
The European Society for Blood and Marrow Transplantation reported on a cohort of 45 adult patients who underwent HCT3 from 2001−2018. They included only patients who underwent HCT3 for relapse or disease progression. The 1- and 2-year OS was 20% and 7% and progression-free survival was 11% and 2%, respectively. On univariate analysis, an improved OS was noted when the Karnofsky score was > 80, an unrelated donor was used, and there was at least one donor change between HCT1 to HCT3. Authors further speculated that patients with AML may be better candidates for HCT3 [13]. In our study, the only patient who survived after HCT3 had ALL; however, this patient received salvage CAR-T treatment for recurrent disease after HCT3 and is now alive more than 7 years after HCT3. The role that disease type plays in determining potential HCT3 benefit remains unclear.
A retrospective study by Kobayashi et al. reported patients from the Japanese national HCT database [14]. In this study, the outcomes of 253 adults undergoing HCT3 for relapsed or refractory acute leukemia between the years of 1994 and 2017 were described. Of the 253 patients, only 20% were in remission at the time of HCT3. OS was 17% and 11% at 2 and 3 years respectively, with median OS of 4.9 months. The authors concluded that remission status and longer duration of remission after HCT2 were the strongest predictors of improved OS. Multivariate analysis revealed that having grade 1 GVHD after HCT2, being > 2 years from HCT2 to relapse, and having ECOG performance score < 2 at time of HCT3 were all associated with improved OS. However, even for patients in CR, a poor performance status was associated with earlier mortality.
Pretransplant comorbidities play an important role in predicting OS after transplant. Sorror et al. previously established that HCT-CI scores ≥ 3 conferred increased risk of death from non-relapse causes [20]. In our study, five of the nine patients had HCT-CI scores ≥ 3 prior to third HCT. It has also been well-established that achievement of deep remission prior to HCT1 provided the most optimal chance for prevention of relapse, and it has been inferred that this remains important for any subsequent HCTs. In our cohort, seven patients were in complete remission by flow cytometry while two had presence of disease (0.07% and refractory disease with resolution of peripheral blasts) at HCT3. The EBMT and Japanese cohorts included approximately 76−80% of patients undergoing HCT3 with refractory or active disease, with 2-year OS of the entire cohort of 7% and 17%, respectively, after HCT3 [13, 14]. In the Japanese registry, a multivariate analysis was performed demonstrating that not being in a CR at time of HCT3 was associated with an increased risk of relapse following HCT3 (HR: 1.89 and 95% CI: 1.15−3.09; p = 0.012). Kobayashi et al. also found that > 2 years from HCT2 to time of relapse was associated with improved OS [14].
Cord blood and haploidentical grafts comprised most donor grafts for HCT3 in our study. It has been suggested that alternative donor sources could offer improved outcomes for subsequent transplants [10, 14, 21,22,23,24]. Rank et al. did find benefit in progression-free and OS with at least one donor change over the course of HCT1 to HCT3 [13]. While our numbers are too small to evaluate any associations with outcomes, all patients in our cohort underwent at least one donor change over the course of three HCTs.
While single center data can be advantageous in providing a study population that has received similar clinical care, our study has several limitations including small sample size, retrospective data collection, and limited data from HCT1 and HCT2 which were performed at outside transplant centers. Additionally, we noted that all patients were of White, non-Hispanic/non-Latino race and ethnicity. As HCT3 is a rare treatment, efforts relating to resource utilization and access of treatments for all patients need to be considered and appropriately offered. However, our small sample size is also indicative of the rarity that third HCTs are performed. This small sample size did not allow the opportunity to perform additional analyses regarding factors predictive of improved survival; however, our results are in line with previously reported outcomes for HCT3.
Though HCT3 may prolong survival in rare cases, we do not know how HCT3 compares to other treatment options including cellular immune therapies or chemotherapy including tyrosine kinase inhibitors, hypomethylating agents, and novel regimens when considering long-term efficacy and quality of life. Additionally, the use of maintenance therapies after HCT3 should be studied and considered for individual patients to sustain a durable remission induced or consolidated by HCT [25, 26].
In summary, HCT3 may prolong survival in rare patients who have relapsed after HCT2. Further longitudinal studies evaluating duration of remission status and quality of life following HCT3 are needed to understand the benefit of this treatment option for patients in this unique situation.
Data availability
No datasets were generated or analysed during the current study.
References
Bar M, Sandmaier BM, Inamoto Y et al (2013) Donor lymphocyte infusion for relapsed hematological malignancies after allogeneic hematopoietic cell transplantation: prognostic relevance of the initial CD3+ T cell dose. Biol Blood Marrow Transplant 19(6):949–957. https://doi.org/10.1016/j.bbmt.2013.03.001
Kekre N, Kim HT, Thanarajasingam G et al (2015) Efficacy of immune suppression tapering in treating relapse after reduced intensity allogeneic stem cell transplantation. Haematologica 100(9):1222–1227. https://doi.org/10.3324/haematol.2015.129650
Chang YJ, Huang XJ (2013) Donor lymphocyte infusions for relapse after allogeneic transplantation: when, if and for whom? Blood Rev 27(1):55–62. https://doi.org/10.1016/j.blre.2012.11.002
Liberio N, Robinson H, Nugent M et al (2019) Single-center experience suggests donor lymphocyte infusion may promote long-term survival in children with high-risk acute lymphoblastic leukemia. Pediatr Blood Cancer 66(11):e27950. https://doi.org/10.1002/pbc.27950
Ciurea SO, Kongtim P, Soebbing D et al (2022) Decrease post-transplant relapse using donor-derived expanded NK-cells. Leukemia 36(1):155–164. https://doi.org/10.1038/s41375-021-01349-4
Smith M, Zakrzewski J, James S, Sadelain M (2018) Posttransplant chimeric antigen receptor therapy. Blood 131(10):1045–1052. https://doi.org/10.1182/blood-2017-08-752121
Zhang C, Wang XQ, Zhang RL et al (2021) Donor-derived CD19 CAR-T cell therapy of relapse of CD19-positive B-ALL post allotransplant. Leukemia 35(6):1563–1570. https://doi.org/10.1038/s41375-020-01056-6
Chen YB, McCarthy PL, Hahn T et al (2019) Methods to prevent and treat relapse after hematopoietic stem cell transplantation with tyrosine kinase inhibitors, immunomodulating drugs, deacetylase inhibitors, and hypomethylating agents. Bone Marrow Transplant 54(4):497–507. https://doi.org/10.1038/s41409-018-0269-3
Dahlberg A, Leisenring W, Bleakley M et al (2019) Prognosis of relapse after hematopoietic cell transplant (HCT) for treatment of leukemia or myelodysplastic syndrome (MDS) in children. Bone Marrow Transplant 54(8):1337–1345. https://doi.org/10.1038/s41409-019-0438-z
Eapen M, Giralt SA, Horowitz MM et al (2004) Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant 34(8):721–727. https://doi.org/10.1038/sj.bmt.1704645
Yaniv I, Krauss AC, Beohou E et al (2018) Second hematopoietic stem cell transplantation for post-transplantation relapsed acute leukemia in children: a retrospective EBMT-PDWP study. Biol Blood Marrow Transplant 24(8):1629–1642. https://doi.org/10.1016/j.bbmt.2018.03.002
Thakar MS, Forman SJ (2009) ASH evidence-based guidelines: is there a role for second allogeneic transplant after relapse? Hematology 2009(1):414–418. https://doi.org/10.1182/asheducation-2009.1.414
Rank A, Peczynski C, Labopin M et al (2021) Feasibility and outcomes of a third allogeneic hematopoietic stem cell transplantation: a retrospective analysis from the acute leukemia working party of the European society for blood and marrow transplantation. Transplant Cell Ther. 27(5):408 el-408 e6. https://doi.org/10.1016/j.jtct.2021.01.025
Kobayashi S, Kanda Y, Konuma T et al (2022) Outcomes of third allogeneic hematopoietic stem cell transplantation in relapsed/refractory acute leukemia after a second transplantation. Bone Marrow Transplant 57(1):43–50. https://doi.org/10.1038/s41409-021-01485-6
Bacigalupo A, Ballen K, Rizzo D et al (2009) Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15(12):1628–1633. https://doi.org/10.1016/j.bbmt.2009.07.004
Gyurkocza B, Sandmaier BM (2014) Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood 124(3):344–353. https://doi.org/10.1182/blood-2014-02-514778
Filipovich AH, Weisdorf D, Pavletic S et al (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 11(12):945–56. https://doi.org/10.1016/j.bbmt.2005.09.004
Przepiorka D, Weisdorf D, Martin P et al (1995) 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15(6):825–828
Sorror ML, Maris MB, Storb R et al (2005) Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106(8):2912–2919. https://doi.org/10.1182/blood-2005-05-2004
Sorror M, Storer B, Sandmaier BM et al (2008) Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer 112(9):1992–2001. https://doi.org/10.1002/cncr.23375
Christopeit M, Kuss O, Finke J et al (2013) Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol 31(26):3259–3271. https://doi.org/10.1200/JCO.2012.44.7961
Ruutu T, de Wreede LC, van Biezen A et al (2015) Second allogeneic transplantation for relapse of malignant disease: retrospective analysis of outcome and predictive factors by the EBMT. Bone Marrow Transplant 50(12):1542–1550. https://doi.org/10.1038/bmt.2015.186
Duncan CN, Majhail NS, Brazauskas R et al (2015) Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant 21(1):151–158. https://doi.org/10.1016/j.bbmt.2014.10.006
Kharfan-Dabaja MA, Labopin M, Polge E et al (2018) Association of second allogeneic hematopoietic cell transplant vs donor lymphocyte infusion with overall survival in patients with acute myeloid leukemia relapse. JAMA Oncol 4(9):1245–1253. https://doi.org/10.1001/jamaoncol.2018.2091
Burchert A, Bug G, Fritz LV et al (2020) Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with flt3-internal tandem duplication mutation (SORMAIN). J Clin Oncol 38(26):2993–3002. https://doi.org/10.1200/JCO.19.03345
DeFilipp Z, Chen YB (2023) How I treat with maintenance therapy after allogeneic HCT. Blood 141(1):39–48. https://doi.org/10.1182/blood.2021012412
Acknowledgements
We would like to thank Cassandra Longsine for her help with manuscript preparation. Support for this study was provided in part by the George and Fay Young Foundation. Kuni Foundation, and from grants P01 CA078902 from National Cancer Institute, National Institutes of Health. This work was also in part supported by the NIH/NCI Cancer Center Support Grant P30 CA015704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health nor their subsidiary Institutes and Center.
Author information
Authors and Affiliations
Contributions
ERC was responsible for acquisition, analysis or interpretation of data and drafting of the manuscript and critical revisions. CS was responsible for study concept and design and acquisition, analysis or interpretation of data. FM, AD, MB & BMS were responsible for the drafting of the manuscript and critical revisions. MST was responsible for study concept and design, acquisition, analysis or interpretation of data, and drafting of the manuscript and critical revisions.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The retrospective study was approved by the Fred Hutchinson Cancer Center Institutional Review Board in accordance with the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cox, E.R., Summers, C., Milano, F. et al. Outcomes of patients undergoing third hematopoietic cell transplantation for hematologic malignancies. Ann Hematol 103, 3737–3743 (2024). https://doi.org/10.1007/s00277-024-05774-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-024-05774-0