Abstract
Patients with FLT3-mutated relapsed or refractory (R/R) acute myeloid leukemia (AML) have a dismal prognosis. Gilteritinib is a FLT3 tyrosine kinase inhibitor (TKI) recently approved for patients with R/R AML. We aimed to characterize real-world data regarding gilteritinib treatment in FLT3-mutated R/R AML and to compare outcomes with matched FLT3-mutated R/R AML patients treated with chemotherapy-based salvage regimens. Twenty-five patients from six academic centers were treated with gilteritinib for FLT3-mutated R/R AML. Eighty percent were treated with a prior intensive induction regimen and 40% of them received prior TKI therapy. Twelve patients (48%) achieved complete response (CR) with gilteritinib. The estimated median overall survival (OS) of the entire cohort was eight (CI 95% 0–16.2) months and was significantly higher in patients who achieved CR compared to those who did not (16.3 months, CI 95% 0–36.2 vs. 2.6 months, CI 95% 1.47–3.7; p value = 0.046). In a multivariate cox regression analysis, achievement of CR was the only predictor for longer OS (HR 0.33 95% CI 0.11–0.97, p = 0.044). Prior TKI exposure did not affect OS but was associated with better event-free survival (HR 0.15 95% CI 0.03–0.71, p = 0.016). An age and ELN-risk matched comparison between patients treated with gilteritinib and intensive salvage revealed similar response rates (50% in both groups); median OS was 9.6 months (CI 95% 2.3–16.8) vs. 7 months (CI 95% 5.1–8.9) in gilteritinib and matched controls, respectively (p = 0.869). In conclusion, in the real-world setting, gilteritinib is effective, including in heavily pre-treated, TKI exposed patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is the most common type of acute leukemia in adults [1]. Despite significant improvements in risk stratification and treatment, disease relapse occurs in up to 50% of patients [2] and is associated with dismal outcome, especially in patients who are considered ineligible for allogeneic stem cell transplantation (alloSCT) [3, 4].
Mutations in the Fms-like tyrosine kinase receptor 3 (FLT3) are found in up to one-third of AML patients (abbreviated as FLT3-mutated AML), with 80% of these mutations being internal tandem duplications (ITD) [5], a trait associated with inferior prognosis due to higher relapse rate, especially in patients with high mutation allelic ratios [6,7,8].
Mutations in FLT3 have been the target for several tyrosine kinase inhibitors (TKI)[9], both in newly diagnosed patients as well as in the relapse or refractory (R/R) disease setting [10,11,12,13,14].
Gilteritinib is a highly potent second-generation, type 1 FLT3 inhibitor that was found to be effective against FLT3-mutated AML [15]. Gilteritinib has recently been approved by the FDA and EMA as monotherapy for patients with R/R FLT 3-mutated AML based on the results of the ADMIRAL phase 3 trial comparing gilteritinib to salvage chemotherapy [16]. Gilteritinib was associated with improved overall survival of 9.3 months vs. 5.5 months in patients in the Standard of care (SOC) group. However, while in the real-world setting most patients with FLT3-mutated AML receive midostaurin during induction based on the results of the RATIFY phase 3 trial [17], only 12% of patients in the ADMIRAL trial treated with FLT3 inhibitor in the upfront setting. Thus, “real-world” analyses of patterns of therapy, response, and safety are warranted.
In this multicenter retrospective cohort study, we aimed to assess and characterize the real-world multi-center data regarding gilteritinib treatment in FLT3-mutated R/R AML and to compare outcomes with patients with R/R AML treated with SOC salvage regimens.
Methods
Study population
We conducted a multicenter nationwide retrospective cohort study in six academic centers in Israel. Gilteritinib treatment was identified by searching the electronical medical records systems of all participating centers and cross referencing these data with the departments’ AML database. We included all consecutive patients with R/R AML who had documented gilteritinib monotherapy treatment as salvage therapy between January 2019 and September 2021. Patient, disease, and treatment characteristics were collected using the electronic medical record system. The study was approved by the Institutional Review Board of each center.
In addition, all patients who were treated upfront with intensive regimens were matched 1:1 with R/R AML patients who were treated with SOC salvage regimens from the surveillance database of the participating centers. Matching was performed according to age and European leukemia network (ELN) 2017 risk score criteria [18]. The selection of these parameters was based on their prognostic value [19, 20]. Patients who were treated in the upfront setting with hypomethylating agents (HMA) and venetoclax combination were seldom considered for salvage regimens other than supportive therapy due to their dismal outlook [21]. Thus, these patients were excluded from the comparative analysis.
Outcomes
Efficacy outcomes included responses according to the recommendations of the European LeukemiaNet and the International working group for assessment and outcomes [18, 22]. Safety data included hematological and nonhematological adverse events (AE), classified according to the CTCAE criteria version 5.0.
Statistics
Categorical variables are presented as numbers and percentages. Continuous variables are presented as mean and standard deviation for normally distributed variables and as median and interquartile range 1–3 (IQR1–3) for non-normally distributed variables. Differences in continuous variables were estimated by t test or Mann–Whitney test, as applicable. Differences in categorical variables were estimated by the Fischer exact test. The probability of OS and EFS were estimated by the Kaplan–Meier method. The log-rank test was used to compare survival distributions.
Cox proportional hazards regression models were fitted to predict effect of covariates on OS and EFS in univariable models. Pre-defined covariates of age and type of induction therapy, as well as covariates with a p value ≤ 0.05 in the univariate model were retained in the cox regression multivariable model for OS and EFS. AlloSCT post gilteritinib use was calculated as time-dependent variable. All statistics were performed with IBM SPSS, version 28.0 (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp) and STATA software version 17.0 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC).
Results
Population
Between January 2019 and September 2021, 25 patients were treated with gilteritinib for R/R AML. The median age at AML diagnosis was 61 years (IQR1–3 47–73). Twenty-two patients (88%) harbored FLT3 mutations at initial diagnosis and 3 patients acquired FLT3 mutations only during relapse—two with FLT3-TKD point mutations and one with FLT-ITD mutation. Induction with standard intensive chemotherapy (“7 + 3”) was given to 20 patients (80%) and 8 patients within this group (40%) were given FLT3 inhibitors as part of their first line therapy (7 midostaurin, 1 quizartinib). Additional patients’ characteristics are presented in Table 1. Overall, 18 patients (72%) achieved CR post induction therapy and of these, ten patients (56%) proceeded to alloSCT in CR1 (Table 1).
The median time from AML diagnosis to gilteritinib treatment was 12 (range 2–51) months and the median number of previous lines of therapy prior to gilteritinib was 2 (range 1–3), with 11 patients (44%) treated with AlloSCT prior to gilteritinib treatment. Gilteritinib was orally administrated in 20 patients (80%) at the full dose of 120 mg, similar to the recommended dose in the ADMIRAL trial, and was reduced to 80 mg in 5 patients (20%) due to cytopenias. The length of each cycle was 28 days and the median number of cycles was 2 (range 1–34).
Response and toxicity
Twelve patients achieved CR (48%), three patients achieved CRi (12%), and ten patients (40%) had stable or progressive disease. After a median time of 7 months post gilteritinib initiation (range 1–34), 23 patients (92%) discontinued treatment. In most cases, this was due to progressive disease or relapse (n = 15, 60%) followed by four patients (16%) due to adverse effects, three patients (12%) who proceeded to alloSCT and did not continue with gilteritinib and one patient (4%) due to completion of two years of treatment (Fig. 1).
The major hematological toxicity was thrombocytopenia (grade ≥ III n = 5, 20% of patients) and the main non-hematological toxicity was grade II elevated liver enzymes (n = 6, 24%, Table 2). No clinically meaningful QT interval prolongations were reported as were no events of differentiation syndrome.
Survival
At the time of last follow up, 7 patients (28%) were alive, with 30-day and 60-day mortality rates of 8% (n = 2) and 28% (n = 7), respectively. Out of 18 death events, 14 (78%) were attributable to leukemia, two (11%) were caused by infection without active disease, one related to acute heart failure post-transplant, and one to acute GVHD.
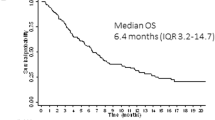
The estimated median OS of patients after gilteritinib initiation was 8 (95%CI 0–16.2) months (Fig. 2A). Median OS was higher in patients treated with intensive induction chemotherapy compared to those treated with induction of HMA-venetoclax (9.6 vs. 1.9 months, p = 0.03). Among patients who achieved CR with gilteritinib, the estimated median OS was significantly higher than patients who did not achieve CR (16.3 (95%CI 0–36.2) months vs. 2.6 months, (95% CI 1.5–3.7); p = 0.046; Fig. 2B). The estimated EFS in the cohort was 3.5 (95% CI 0–7.9) months, with higher median EFS among patients who achieved CR compared to those who did not (7 (95%CI 0–14.2) vs. 1.86 (95%CI 0.77–3.0) months, p = 0.045).
In univariate analysis, initial intensive induction therapy vs. low-intensity regimen predicted better OS with gilteritinib treatment (HR 0.33, CI 95% 0.11–0.98, p = 0.045). Treatment with a FLT3 inhibitor during first induction and achievement of CR after gilteritinib treatment were associated with better OS, albeit with borderline significance (HR 0.29, 95% CI 0.08–1.01, p = 0.052 and 0.37, 95% CI 0.14–1.03 p = 0.057). Age, type of AML (de novo vs. secondary AML), ECOG score, ELN risk category, number of previous lines of treatment, time from first therapy to gilteritinib treatment, and alloSCT (prior to gilteritinib or following gilteritinib) did not significantly impact OS after gilteritinib use (Table 3). In a multivariate model that included CR post gilteritinib and FLT3-targeted therapy during induction, as well as pre-defined covariates—age, and type of induction therapy—only CR achievement post gilteritinib retained its predictive value (HR 0.33 95% CI 0.11–0.97, p = 0.044).
In a univariate EFS analysis, treatment with FLT3 inhibitor during induction therapy was predictive of better EFS (HR 0.14, 95% CI 0.03–0.61, p = 0.009). Intensive induction therapy and achieving CR post gilteritinib were associated with better EFS, albeit borderline significance (HR 0.35, 95% CI 0.12–1.0, p = 0.05 and HR 0.39, 95% CI 0.15–1.02, p = 0.054, respectively). In a multivariate model that included CR post gilteritinib and FLT3-targeted therapy during induction, as well as pre-defined covariates—age, and type of induction therapy—CR achievement as well as prior treatment with FLT3 inhibitor were associated with better EFS (HR 0.37 95% CI 0.12–0.94, p = 0.037 and HR 0.15 95% CI 0.03–0.71, p = 0.016).
Matched analysis of gilteritinib vs. intensive salvage regimens
In order to gain insight into comparative efficacy of gilteritinib in the real-world setting, we conducted a matched analysis that focused on the 20 R/R patients that were initially treated with intensive induction. Patients were matched by age and ELN risk score for the purposes of this analysis.
There were no statistical differences between gilteritinib and SOC groups in terms of baseline characteristics (supplementary S1Table 1) except for the number of previous lines of therapy and the duration from diagnosis to salvage therapy. All the patients in the SOC salvage therapy group (SOC group) were treated with one previous line of therapy, vs. median of two lines of therapy (range 1–3) in the gilteritinib group (p = 0.047). As a result, the duration between diagnosis and salvage therapy was longer in the gilteritinib group as compared to the SOC group median (median 11 months, IQR1–3 4–37 vs. median 5 months, IQR1–3 1–9, respectively, p = 0.003).
Among the SOC group, 50% (n = 10) were treated with FLT3 TKI during the induction treatment. Salvage consisted of high dose cytarabine and anthracycline in 16 patients (80%), HMA and venetoclax in two patients, one patient was treated with HMA only, and one patient was treated for refractory AML with sequential alloSCT. Specific regimen details and other SOC group characteristics are shown in Table S1.
Response rates were similar in both groups with ten patients (50%) achieving CR in both groups (p = 1). Likewise, 9 patients (45%) in both the gilteritinib group and in the SOC group proceeded to alloSCT.
The OS among the gilteritinib group was 9.6 (CI 95% 2.3–16,8) months as compared to 7 months (CI 95% 5.1–8.9) in the matched controls (log rank p = 0.869, Fig. 3A). Similarly, the EFS was 5.1 months (CI 95% 0–10.3) and 3.3 months (CI 95% 0–7.3, Fig. 3B) in the gilteritinib and the SOC group, respectively (log rank p = 0.607).
Discussion
Herein, we report the results of real-world data regarding treatment with gilteritinib for R/R AML. The median OS in our cohort was 8 months (CI 95% 0–16.2) and is largely comparable to the results demonstrated in the ADMIRAL trial. Achieving CR after gilteritinib and initial treatment with TKI during induction therapy predicted better EFS following gilteritinib treatment, whereas only the former was predictive of OS as well. In a matched analysis of gilteritinib vs. SOC intensive regimens, the median OS was slightly longer with gilteritinib but did not reach statistical significance.
The outcome of patients with R/R FLT3-mutated AML is extremely dismal. Analysis of a French registry demonstrated a median survival of 7 to 8 months for R/R patients with FLT3-ITD in the era before second-generation FLT3 inhibitors were utilized for this indication [23, 24]. The ADMIRAL trial demonstrated that monotherapy with gilteritinib was associated with more than doubling of the remission rates for patients with R/R FLT3-mutated AML as compared to salvage chemotherapy (54.3% vs. 21.8%, respectively). This was translated into to significantly improved survival in patients treated with gilteritinib (median overall survival of 9.3 vs. 5.6 months, respectively; HR 0.64 (95% CI, 0.49–0.83)) and was the basis for the approval of gilteritinib for this indication by the FDA and EMA in 2018 and 2019, respectively. The results of a similar phase 3, randomized open-label study in Asia (the COMMODORE trial) were recently reported and showed similar superiority for gilteritinib over SOC salvage chemotherapy [25].
Patients in our analysis differed from those in the ADMIRAL trial. One-third of patients analyzed in the current study (and 42% of intensively treated patients) received midostaurin during induction as compared to only 12% of patients in the ADMIRAL trial. Furthermore, the median number of previous lines of therapy prior to gilteritinib was 2 (range 1–3) in our analysis, over one-third of patients had ELN adverse risk disease (as compared to only 10% in the ADMIRAL), and 44% of patients in our analysis received gilteritinib for post-alloSCT relapse as compared to 20% in the registration trial. In this heavily pre-treated patient population, the survival was at a median of 8 months. Survival was estimated at 9.6 months for patients that were initially deemed eligible for intensive therapy and was higher (though not statistically different) from the 7 months reported in matched controls. Of note, this match analysis focus on intensively treated patients with the gilteritinib group having more previous lines of therapy that may account for the blunted differenced in outcome between the groups as compared to the ADMIRAL trial.
Patients who responded and achieved remission with gilteritinib derived the most benefit from therapy with an estimated median OS of 16.3 months and predictors of response for this patient population may aid in guiding therapy assignment.
The relative efficacy of gilteritinib in patients previously exposed to FLT3 inhibitors in our analysis is reassuring and is in line with a recent retrospective analysis [26]. In addition, A previous combined analysis of the CHRYSALIS and ADMIRAL clinical trials demonstrate that patients previously exposed to FLT3 inhibitors derived benefit from gilteritinib albeit to a lesser extent from of those not previously exposed to FLT3 inhibitors [27].
The encouraging response rates in the intensively treated cohort are in stark contrast to the very poor survival in patients that were sequenced gilteritinib after failure of HMA and venetoclax combination (median OS 1.9 months). These data are in line with the poor survival reported for the subgroup of patients treated with low-intensity approaches in the ADMIRAL trial and with previous outcomes reported for patients with HMA-venetoclax failure [21]. Based on our data, it seems that gilteritinib does not effectively salvage patient in this clinical setting. Combining gilteritinib to venetoclax with or without HMA’s seems to have synergistic effects that result in high response rates even in patients previously exposed to FLT3 inhibitors. Such combinations may represent an attractive approach for low intensity treated FLT3-mutated patients [28,29,30].
This study is limited by its retrospective design and the relatively small number of patients. Nonetheless, it represents the first matched analysis in a real-world setting to our knowledge and provides insights as to treatment and response patterns with current therapeutic approaches for patients with FLT3-mutated AML.
In summary, gilteritinib is effective and well-tolerated in our patient cohort. In our analysis, patients seemed to receive gilteritinib at advanced time points in the course of therapy as compared to the clinical trials and earlier sequencing of this agent in the FLT3-mutated R/R setting may potentially optimize outcomes for this agent. Combination strategies for gilteritinib with venetoclax and other targeted approaches may represent another way to further improve responses and should be sought within clinical trials.
Change history
23 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00277-022-04929-1
References
American Cancer Society (2021) American Cancer Society: Cancer Facts and Figures 2021. . https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf. Accessed 2021/12/22 2021
Dohner H, Weisdorf DJ, Bloomfield CD (2015) Acute myeloid leukemia. N Engl J Med 373(12):1136–1152. https://doi.org/10.1056/NEJMra1406184
Bethesda MNCI, DCCPS, Surveillance Research Program, 2020. Surveillance, epidemiology, and end results program: cancer stat facts: leukemia—acute myeloid leukemia (AML). https://seer.cancer.gov/statfacts/html/amyl.html. Accessed 12/22/2021 2021
Estey E (2000) Treatment of relapsed and refractory acute myelogenous leukemia. Leukemia 14(3):476–479. https://doi.org/10.1038/sj.leu.2401568
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F, Bolli N, Gundem G, Van Loo P, Martincorena I, Ganly P, Mudie L, McLaren S, O’Meara S, Raine K, Jones DR, Teague JW, Butler AP, Greaves MF, Ganser A, Dohner K, Schlenk RF, Dohner H, Campbell PJ (2016) Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374(23):2209–2221. https://doi.org/10.1056/NEJMoa1516192
Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, Carroll AJ, Mrózek K, Vardiman JW, George SL, Kolitz JE, Larson RA, Bloomfield CD, Caligiuri MA (2001) Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res 61(19):7233–7239
D. Kottaridis P, Gale RE, Linch DC, (2003) Flt3 mutations and leukaemia. Br J Haematol 122(4):523–538. https://doi.org/10.1046/j.1365-2141.2003.04500.x
Thiede C, Steudel C, Mohr B, Schaich M, SchäKel U, Platzbecker U, Wermke M, BornhäUser M, Ritter M, Neubauer A, Ehninger G, Illmer T (2002) Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 99(12):4326–4335. https://doi.org/10.1182/blood.v99.12.4326
Smith CC, Wang Q, Chin C-S, Salerno S, Damon LE, Levis MJ, Perl AE, Travers KJ, Wang S, Hunt JP, Zarrinkar PP, Schadt EE, Kasarskis A, Kuriyan J, Shah NP (2012) Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 485(7397):260–263. https://doi.org/10.1038/nature11016
Daver N, Schlenk RF, Russell NH, Levis MJ (2019) Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia 33(2):299–312. https://doi.org/10.1038/s41375-018-0357-9
Cortes J, Perl AE, Döhner H, Kantarjian H, Martinelli G, Kovacsovics T, Rousselot P, Steffen B, Dombret H, Estey E, Strickland S, Altman JK, Baldus CD, Burnett A, Krämer A, Russell N, Shah NP, Smith CC, Wang ES, Ifrah N, Gammon G, Trone D, Lazzaretto D, Levis M (2018) Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label multicentre single-arm phase 2 trial. Lancet Oncol 19(7):889–903. https://doi.org/10.1016/s1470-2045(18)30240-7
Cortes JE, Kantarjian HM, Kadia TM, Borthakur G, Konopleva M, Garcia-Manero G, Daver NG, Pemmaraju N, Jabbour E, Estrov Z, Ramachandran A, Paradela J, Pond B, Ravandi F, Vusirikala M, Patel PA, Levis MJ, Perl AE, Andreeff M, Collins R (2016) Crenolanib besylate, a type I pan-FLT3 inhibitor, to demonstrate clinical activity in multiply relapsed FLT3-ITD and D835 AML. J Clin Oncol 34((15_suppl)):7008–7008. https://doi.org/10.1200/JCO.2016.34.15_suppl.7008
Stone RM, Deangelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, Grandin W, Lebwohl D, Wang Y, Cohen P, Fox EA, Neuberg D, Clark J, Gilliland DG, Griffin JD (2005) Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 105(1):54–60. https://doi.org/10.1182/blood-2004-03-0891
Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ, Faderl S, Verstovsek S, Mathews S, Andreeff M, Cortes JE (2011) Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica 96(1):62–68. https://doi.org/10.3324/haematol.2010.030452
Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, Claxton D, Erba HP, Gill S, Goldberg S, Jurcic JG, Larson RA, Liu C, Ritchie E, Schiller G, Spira AI, Strickland SA, Tibes R, Ustun C, Wang ES, Stuart R, Röllig C, Neubauer A, Martinelli G, Bahceci E, Levis M (2017) Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol 18(8):1061–1075. https://doi.org/10.1016/s1470-2045(17)30416-3
Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, Montesinos P, Baer MR, Larson RA, Ustun C, Fabbiano F, Erba HP, Di Stasi A, Stuart R, Olin R, Kasner M, Ciceri F, Chou WC, Podoltsev N, Recher C, Yokoyama H, Hosono N, Yoon SS, Lee JH, Pardee T, Fathi AT, Liu C, Hasabou N, Liu X, Bahceci E, Levis MJ (2019) Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med 381(18):1728–1740. https://doi.org/10.1056/NEJMoa1902688
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede C, Prior TW, Dohner K, Marcucci G, Lo-Coco F, Klisovic RB, Wei A, Sierra J, Sanz MA, Brandwein JM, de Witte T, Niederwieser D, Appelbaum FR, Medeiros BC, Tallman MS, Krauter J, Schlenk RF, Ganser A, Serve H, Ehninger G, Amadori S, Larson RA, Dohner H (2017) Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377(5):454–464. https://doi.org/10.1056/NEJMoa1614359
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Lowenberg B, Bloomfield CD (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447. https://doi.org/10.1182/blood-2016-08-733196
Chevallier P, Labopin M, Turlure P, Prebet T, Pigneux A, Hunault M, Filanovsky K, Cornillet-Lefebvre P, Luquet I, Lode L, Richebourg S, Blanchet O, Gachard N, Vey N, Ifrah N, Milpied N, Harousseau JL, Bene MC, Mohty M, Delaunay J (2011) A new leukemia prognostic scoring system for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia 25(6):939–944. https://doi.org/10.1038/leu.2011.25
Breems DA, Van Putten WLJ, Huijgens PC, Ossenkoppele GJ, Verhoef GEG, Verdonck LF, Vellenga E, De Greef GE, Jacky E, Van Der Lelie J, Boogaerts MA, Löwenberg B (2005) Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol 23(9):1969–1978. https://doi.org/10.1200/jco.2005.06.027
Abhishek M, Caitlin RR, Jorge EC, Naveen P, Naval GD, Farhad R, Guillermo G-M, Gautam B, Kiran N, Maro O, Nicholas JS, Yesid A, Tapan MK, Koichi T, Musa Y, Nitin J, Steven K, Guillermo Montalban B, Koji S, Michael A, Prithiviraj B, Alessandra F, Ghayas CI, Elias JJ, Lucia M, Philip AT, Sa W, Sergej K, Sherry AP, Jing N, Wei Q, John SW, Hagop MK, Courtney DD, Marina YK (2020) Outcomes of relapsed or refractory acute myeloid leukemia after frontline hypomethylating agent and venetoclax regimens. Haematologica 106(3):894–898. https://doi.org/10.3324/haematol.2020.252569
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD (2003) Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21(24):4642–4649. https://doi.org/10.1200/jco.2003.04.036
Bertoli S, Dumas P-Y, Bérard E, Largeaud L, Bidet A, Delabesse E, Tavitian S, Gadaud N, Leguay T, Leroy H, Rieu J-B, Vial J-P, Vergez F, Lechevalier N, Luquet I, Klein E, Sarry A, De Grande A-C, Récher C, Pigneux A (2020) Outcome of relapsed or refractory FLT3-mutated acute myeloid leukemia before second-generation FLT3 tyrosine kinase inhibitors: a Toulouse-Bordeaux DATAML Registry Study. Cancers 12(4):773. https://doi.org/10.3390/cancers12040773
Dumas P-Y, Bertoli S, Bérard E, Largeaud L, Bidet A, Delabesse E, Leguay T, Leroy H, Gadaud N, Rieu JB, Vial J-P, Vergez F, Lechevalier N, Luquet I, Klein E, Sarry A, De Grande A-C, Pigneux A, Récher C (2020) Real-world outcomes of patients with refractory or relapsed FLT3-ITD acute myeloid leukemia: a Toulouse-Bordeaux DATAML Registry Study. Cancers 12(8):2044. https://doi.org/10.3390/cancers12082044
Wang J, Jiang B, Li J, Liu L, Du X, Jiang H, Hu J, Yuan M, Sakatani T, Kadokura T, Takeuchi M, Izuka S, Girshova L, Tan J, Bondarenko SN, Wong LL, Khuhapinant A, Martynova E, Hasabou N, Tiu RV (2021) Gilteritinib versus salvage chemotherapy for relapsed/refractory FLT3-mutated acute myeloid leukemia: a phase 3, randomized, multicenter, open-label trial in Asia. Blood 138(Supplement 1):695–695. https://doi.org/10.1182/blood-2021-145436
Numan Y, Rahman ZA, Grenet J et al (2022) Gilteritinib clinical activity in relapsed/refractory FLT3 mutated acute myeloid leukemia previously treated with FLT3 inhibitors. Am J Hematol 97(3):322–328. https://doi.org/10.1002/ajh.26447
Perl AE, Altman JK, Hosono N, Montesinos P, Podoltsev NA, Martinelli G, Smith CC, Levis M, Röllig C, Groß-Langenhoff M, Hasabou N, Lu Q, Tiu RV (2020) Clinical outcomes in patients with relapsed/refractory acute myeloid leukemia treated with Gilteritinib who received prior Midostaurin or Sorafenib. Blood 136(Supplement 1):22–23. https://doi.org/10.1182/blood-2020-136395
Daver N, Altman JK, Maly J, Levis M, Ritchie E, Litzow M, McCloskey JK, Smith CC, Schiller GJ, Bradley T, Tiu RV, Hong W-J, Tong B, Qin Q, Dilley K, Perl AE (2020) Efficacy and safety of Venetoclax in combination with Gilteritinib for relapsed/refractory FLT3-mutated acute myeloid leukemia in the expansion Cohort of a Phase 1b Study. Blood 136(Supplement 1):20–22. https://doi.org/10.1182/blood-2020-139705
Ma J, Zhao S, Qiao X, Knight T, Edwards H, Polin L, Kushner J, Dzinic SH, White K, Wang G, Zhao L, Lin H, Wang Y, Taub JW, Ge Y (2019) Inhibition of Bcl-2 synergistically enhances the antileukemic activity of Midostaurin and Gilteritinib in preclinical models of FLT3-mutated acute myeloid leukemia. Clin Cancer Res 25(22):6815–6826. https://doi.org/10.1158/1078-0432.CCR-19-0832
Nicholas J. Short CDD, Naval Daver, MD et al. 2021 A triplet combination of Azacitidine, Venetoclax and Gilteritinib for patients with FLT3-mutated acute myeloid leukemia: results from a phase I/II study. In: American society of Hematology annual meeting, https://ash.confex.com/ash/2021/webprogram/Paper153571.html
Funding
There was no funding support for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: This article was originally published with a missing author (Dr. Israel Henig).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimony, S., Canaani, J., Kugler, E. et al. Gilteritinib monotherapy for relapsed/refractory FLT3 mutated acute myeloid leukemia: a real-world, multi-center, matched analysis. Ann Hematol 101, 2001–2010 (2022). https://doi.org/10.1007/s00277-022-04895-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04895-8