Abstract
Daratumumab (DARA) is a human IgG-K monoclonal antibody (MoAb) targeting CD38 that is approved alone or in combination with bortezomib and dexamethasone or lenalidomide and dexamethasone for relapsed or refractory MM (RRMM) in patients previously exposed or double refractory to proteasome inhibitors (PI) and immunomodulatory drugs (IMiDs). However, there are limited data on its clinical activity and tolerability in real-world patients. Therefore, in the present study, we aim to determine the efficacy and toxicity profile of daratumumab in a real-life setting. In this study, we report the experience of the multiple myeloma GIMEMA Lazio Group in 62 relapsed/refractory MM patients treated with daratumumab as monotherapy who had previously received at least two treatment lines including a PI and an IMiDs or had been double refractory. Patients received DARA 16 mg/kg intravenously weekly for 8 weeks, every 2 weeks for 16 weeks, and every 4 weeks until disease progression or unacceptable toxicity. The overall response rate to daratumumab was 46%. Median progression-free survival (PFS) and overall survival reached 2.7 and 22.4 months, respectively. DARA was generally well tolerated; however, 2 patients interrupted their therapy due to adverse events. Present real-life experience confirms that DARA monotherapy is an effective strategy for heavily pre-treated and refractory patients with multiple myeloma, with a favorable safety profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment of multiple myeloma (MM) patients (pts) has radically changed over the last years following the introduction of next-generation proteasome inhibitors (PI) and immunomodulatory derivative (IMiDs). Almost all pts eventually relapse despite their responses to PI, IMiDs, or both. In the last years, one further therapeutic option for MM patients is represented by daratumumab (DARA), a human IgG1-Kmonoclonal antibody (MoAb) recently introduced in the treatment of relapsed or relapsed and refractory multiple myeloma (RRMM) that binds with high affinity to a unique epitope on a cluster of differentiation 38 (CD38). DARA is a targeted immunotherapy that induces lysis of CD38-overexpressing tumor cells by a number of mechanisms, including complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, and antibody-dependent cellular phagocytosis, through the activation of complement proteins, natural killer (NK) cells, and macrophages, respectively [1, 2]. DARA was approved by the US Food and Drug Administration (FDA) as a single agent for advanced MM, based on the results of the phase II trial SIRIUS [3], in which, even in a setting of highly pre-treated MM patients (5median treatment lines), they showed a considerable rate of response with prolongation of progression-free survival (PFS) and overall survival (OS) and a favorable safety profile.
Since the effectiveness of any single drug should be further confirmed by further trials and clinical practice, in the present study, we report the experience of the multiple myeloma GIMEMA Lazio group in 62 highly pre-treated MM pts who received DARA as monotherapy.
Patients and methods
The basic demographics and the clinical and laboratory features of the 62 patients included in the study are summarized in Table 1. Thirty-two (52%) pts had an abnormal serum free light-chain ratio (FLC ratio > 10). Four patients (6%) had extramedullary disease (3 had a bone plasmacytoma and 1 a cutaneous plasmacytoma); 45 (72%) pts had bone involvement. Cytogenetic features are not available. Thirty-three patients (53%) had previously received autologous transplant; 57 (92%) received previous bortezomib, 21 (34%) carfilzomib, 30 (48%) thalidomide, 60 (96%) lenalidomide, and 22 pomalidomide (36%). Thirty-nine (63%) pts were refractory to the last line of therapy. All patients previously treated at least with two lines of therapy, including a PI and an IMiDs or double refractory, received DARA 16 mg/kg intravenously. Treatment response was evaluated according to the International Myeloma Working Group (IMWG) criteria. Patients who received at least one dose of study drug were included in the statistical analysis. Patients’ and disease characteristics were summarized by means of cross-tabulations for categorical variables or by quintiles for continuous variables. OS (time elapsed from treatment start to death) and PFS (time from treatment start to progression or death) were calculated using the Kaplan-Meier product limit estimator. Medians were presented with a 2-sided 95% confidence interval based on the non-parametric method. All analyses were performed using the R (R Foundation for Statistical Computing, Vienna, Austria) system software.
Results
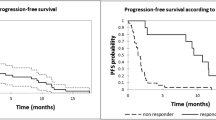
After a median follow-up from diagnosis of 60.6 months (range 7.0–206.0) and a median of 3 previous lines of therapy (range 2–8), pts received a median of 3 cycles (range 1–18) of DARA and a median of 10 doses (range 1–29). The overall response rate was 42% (26 pts); in particular, 2 (3%) pts obtained a CR, 5 (8%) pts a VGPR, 19 (31%) pts a PR, and 16 (28%) pts a SD, while 15 (24%) pts presented a PD. After a median follow-up of 23.7 months (range 21.9–27.1), 6 (10%) were still in response and alive after stopping DARA, 25 (40%) were alive and performed a new therapy, 31 (50%) died, 1 in PR due to post-allograft GVHD, 27 experienced a PD (1 CR, 3 VGPR, 13 PR, and 10 SD), and 2 died for cardiac disease and 1 for infectious complications. Overall, 31 (50%) pts are still alive and 31 (50%) died. The median time to response, duration of response, progression-free survival, and overall survival were 1.5 months (range 1.0–6.0), 2.5 months (95% CI, 0.8–4.1), 2.7 months (95% CI, 2.3–4.6), and 22.4 months (95% CI, 13.3–NA), respectively. PFS, OS, and DOR are represented in Fig. 1. DARA was well tolerated; the most common adverse events, of grade 3/4, included anemia in 9 (15.7%) pts, thrombocytopenia in 7 (12.2%) pts, and neutropenia in 3 (5.2%) pts. Common SAEs included pneumonia in 12 (20.9%) pts, upper respiratory tract infection in 9 (15.7%) pts, and fatigue in 8 (14%) pts, which did not lead to treatment discontinuation but only to dose delay. Infusion-related reactions (IRRs) occurred in 9 (15.7%) pts, grade I–II 5 pts, grade III 4 pts, during the first three infusions that lead to treatment discontinuation in 2 pts. The most common IRRs were dyspnea (9.4%), nasal congestion (7.2%), and cough (7.1%). The primary reasons for treatment discontinuation included progressive disease (93.6%), AE (3.2%), and death (3.2%).
Discussion
Two trials, the GEN501 and SIRIUS studies, initially evaluated the use of DARA monotherapy [3, 4]. A combined analysis of these two monotherapy studies was performed and included 148 patients [5, 6]. In this combined analysis, the median time to response in patients with PR or better was 0.95 months, and the ORR was 31.1% (95% confidence interval (CI), 23.7–39.2%). The median duration of response was 7.6 months. The median OS for the group was 20.1 months, with a 1-year OS rate of 69% [7]. Compared to these two latter studies, the present patient population series, although constituted by a limited number of MM patients, showed similar clinic-biological features, with the exception of a lower median number of previous lines of therapies that were 3 (range 2–8) in our study compared to the 5 (range 2–14) courses in the study of Usmani et al. [6]. Our results, compared favorably with those of the other studies, are reported in Table 2. ORR was higher in our series compared to that reported in the GEM501 and Sirius studies (42% vs 31.1%), due to an increased number of patients achieving partial responses (31% vs 17.6%). Likely, this difference may be explained by the lower number of previous therapies in our MM patients. We believe that the present data are relevant because they were achieved in a real-world setting in contrast to clinical trials.
In our study, none of the patients achieved a sCR and 2 (3%) patients achieved a CR of disease. These findings are clearly inferior to those reported in the CASTOR and POLLUX study in which the rate of sCR and CR were, respectively, 4.6% and 14.6% and 18.1% and 24.9% (Table 2). As known, in these latter two studies, DARA was combined with IP and IMIDs, respectively, and the impressive results not only in terms of sCR and CR but also in terms of PFS and OS clearly demonstrated the need to combine DARA with bortezomib or lenalidomide to get deep and lasting responses. In addition, among the 31 patients alive, 25 (81%) continued on further therapies. These data further confirm the suggestion that perhaps DARA improves responses to future lines of therapy by modifying the immune environment [12, 13] and interaction with bone-marrow stromal cells [14]. Regarding safety profile, DARA was well tolerated and treatment discontinuation due to toxicity occurred only in 2 of the 62 pts. Our result shows a lower incidence of hematological toxicity and a reduction of IRRs grades 1–2 (8.7% vs 48%), but an increase of IRR grades 3–4 that leads to treatment discontinuation in two pts. More recently, daratumumab in combination with different standards of care has been approved for both relapsed/refractory and new MM patients. In combination with bortezomib and dexamethasone (DVd) or lenalidomide and dexamethasone (DRd), daratumumab induced rapid, deep, and durable responses, reducing the risk of disease progression or death in relapsed/refractory MM patients [15, 16]. However, not all patients are able to receive treatment based on triplets due to higher AE reported used triplets vs doublet regimens. In this regard, DARA as a monotherapy could be considered a useful therapeutic option too.
Conclusions
The present findings further confirm in a real-life setting that DARA monotherapy is an effective strategy for heavily pre-treated and refractory pts with multiple myeloma, with a favorable safety profile. This treatment option should be considered for those MM pts not eligible for triplets or who are refractory to proteasome inhibitors and IMiDs, who would be otherwise left for palliative care.
References
de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW, Mutis T, Bleeker WK, Anderson KC, Lokhorst HM, van de Winkel JG, Parren PW (2011) Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840–1848
Overdijk MB, Verploegen S, Bogels M et al (2015) Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 7:311–321
Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, Belch A, Krishnan A, Vescio RA, Mateos MV, Mazumder A, Orlowski RZ, Sutherland HJ, Blade J, Scott EC, Oriol A, Bardeja J, Gharibo M, Stevens DA, Le Blanc R, Sebag M, Callander N, Jakubowiak A, White D, de la Rubia J, Richardson PG, Lisby S, Feng H, Uhlar CM, Khan I, Ahmadi T, Voorhees PM (2016) Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 387:1551–1560
Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, van de Donk NWCJ, Ahmadi T, Khan I, Uhlar CM, Wang J, Sasser AK, Losic N, Lisby S, Basse L, Brun N, Richardson PG (2015) Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 373:1207–1219
Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, Lokhorst HM, Voorhees PM, Richardson PG, Chari A, Sasser AK, Axel A, Feng H, Uhlar CM, Wang J, Khan I, Ahmadi T, Nahi H (2016) Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 128:37–44
Usmani SZ, Diels J, Ito T, Mehra M, Khan I, Lam A (2017) Daratumumab monotherapy compared with historical control data in heavily pretreated and highly refractory patients with multiple myeloma: an adjusted treatment comparison. Am J Hematol 92:E146–E152
Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, Haessler J, Feather J, Hoering A, Moreau P, LeLeu X, Hulin C, Klein SK, Sonneveld P, Siegel D, Blade J, Goldschmidt H, Jagannath S, Miguel JS, Orlowski R, Palumbo A, Sezer O, Rajkumar SV, Durie BG, International Myeloma WorkingGroup (2012) Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia 26:149–157
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P, CASTOR investigators (2016) Daratumumab, Bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375(8):754–766
Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, Masszi T, Lauri B, Levin MD, Bosi A, Hungria V, Cavo M, Lee JJ, Nooka AK, Quach H, Lee C, Barreto W, Corradini P, Min CK, Scott EC, Chanan-Khan AA, Horvath N, Capra M, Beksac M, Ovilla R, Jo JC, Shin HJ, Sonneveld P, Soong D, Casneuf T, Chiu C, Amin H, Qi M, Thiyagarajah P, Sasser AK, Schecter JM, Mateos MV (2018) Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica 103(12):2079–2087
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki K, Plesner T, Yoon SS, Ben Yehuda D, Richardson PG, Goldschmidt H, Reece D, Lisby S, Khokhar NZ, O'Rourke L, Chiu C, Qin X, Guckert M, Ahmadi T, Moreau P, POLLUX investigators (2016) Daratumumab, Lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375(14):1319–1331
Dimopoulos MA, San-Miguel J, Belch A, White D, Benboubker L, Cook G, Leiba M, Morton J, Ho PJ, Kim K, Takezako N, Moreau P, Kaufman JL, Sutherland HJ, Lalancette M, Magen H, Iida S, Kim JS, Prince HM, Cochrane T, Oriol A, Bahlis NJ, Chari A, O'Rourke L, Wu K, Schecter JM, Casneuf T, Chiu C, Soong D, Sasser AK, Khokhar NZ, Avet-Loiseau H, Usmani SZ (2018) Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica 103(12):2088–2096. https://doi.org/10.3324/haematol.2018.194282Epub 2018 Sep 20
Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, Syed K, Liu K, van de Donk NWCJ, Weiss BM, Ahmadi T, Lokhorst HM, Mutis T, Sasser AK (2016) Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128:384–394
Chatterjee S, Daenthanasanmak A, Chakraborty P, Wyatt MW, Dhar P, Selvam SP, Fu J, Zhang J, Nguyen H, Kang I, Toth K, Al-Homrani M, Husain M, Beeson G, Ball L, Helke K, Husain S, Garrett-Mayer E, Hardiman G, Mehrotra M (2018) CD38-NAD+Axis regulates immunotherapeutic anti-tumor T cell response. Cell Metab 27:85–100.e8
Marlein CR, Piddock RE, Mistry JJ, Zaitseva L, Hellmich C, Horton RH, Zhou Z, Auger MJ, Bowles KM, Rushworth SA (2019 May 1) CD38-driven mitochondrial trafficking promotes bioenergetic plasticity in multiple myeloma. Cancer Res 79(9):2285–2297
Nooka AK, Kastritis E, Dimopoulos MA, Lonial S (2015) Treatment options for relapsed and refractory multiple myeloma. Blood 125:3085–3099
Boyle EM, Leleu X, Petillon MO, Karlin L, Doyen C, Demarquette H, Royer B, Macro M, Moreau P, Fostier K, Marie-Lorraine C, Zarnitsky C, Perrot A, Herbaux C, Poulain S, Manier S, Beauvais D, Walker BA, Wardell CP, Vincent L, Frenzel L, Caillon H, Susanna S, Dejoie T, Avet-Loiseau H, Mohty M, Facon T, IFM2014–04 investigators (2019) Daratumumab and dexamethasone is safe and effective for triple refractory myeloma patients: final results of the IFM 2014–04 (Etoile du Nord) trial. Br J Haematol 187:319–327
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
-
F. Vozella has received grants/speaker honorarium from Amgen, Celgene, Takeda;
-
Siniscalchi declares that she has no conflict of interest;
-
M. Rizzo declares that she has no conflict of interest;
-
T. Za declares that he has no conflict of interest;
-
G. Antolino has received grants/speaker honorarium from Amgen, BMS, Celgene, Janssen-Cilag, Takeda;
-
U. Coppetelli declares that he has no conflict of interest;
-
Piciocchi declares that he has no conflict of interest;
-
Andriani has received grants/speaker honorarium from Abbvie, Celgene, Janssen-Cilag, Takeda;
-
O. Annibali has received grants/speaker honorarium from Amgen, Celgene, Janssen-Cilag, Roche, Takeda;
-
L. De Rosa has received grants/speaker honorarium from Amgen, Celgene, Janssen-Cilag, Roche, Takeda;
-
G. Cimino declares that he has no conflict of interest;
-
G. La Verde has received grants/speaker honorarium from Amgen, BMS, Celgene, Janssen-Cilag, Takeda;
-
V. De Stefano has received grants/speaker honorarium from AIFA, Alexion, Amgen, Bayer, Catholic University School of Medicine, Celgene, Janssen-Cilag, Novartis, Takeda;
-
M. Cantonetti has received grants/speaker honorarium from Gilead, Incyte, Janssen-Cilag, Kyowa Kirin, Mundipharma, Roche, Sandoz, Takeda, Vifor;
-
T. Caravita di Toritto has received grants/speaker honorarium from Amgen, Celgene, Janssen-Cilag, Takeda;
-
M.T. Petrucci has received grants/speaker honorarium from Amgen, Celgene, Janssen-Cilag, Takeda.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vozella, F., Siniscalchi, A., Rizzo, M. et al. Daratumumab in multiple myeloma: experience of the multiple myeloma GIMEMA Lazio group. Ann Hematol 100, 1059–1063 (2021). https://doi.org/10.1007/s00277-020-04374-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04374-y