Abstract
Prognostic indices combining several clinical and laboratory parameters have been proposed for prognostication in chronic lymphocytic leukemia (CLL). Recently, international consortium on CLL proposed an international prognostic index (CLL-IPI) integrating clinical, molecular, and genetic parameters. The present study was designed to evaluate the reproducibility of CLL-IPI in Indian CLL cohort. The prognostic ability of CLL-IPI in terms of overall survival (OS) and time to first treatment (TTFT) was investigated in treatment-naive CLL patients and also compared with other existing prognostic scores. For assigning scores, clinical and laboratory details were obtained from medical records, and IGHV gene mutation status, β2-microglobulin levels, and copy number variations were determined using c-DNA, ELISA, and multiplex ligation-dependent probe amplification (MLPA), respectively. The scores were generated as per the weighted grades assigned to each variable involved in score categorization. The predictive value of prognostic models was assessed and compared using Harrell’s C-index and Akaike’s information criterion (AIC). Stratification of patients according to CLL-IPI yielded significant differences in terms of OS and TTFT (p < 0.001). Comparative assessment of scores for OS suggested better performance of CLL-IPI (C = 0.64, AIC = 740) followed by Barcelona–Brno (C = 0.61, AIC = 754) and MDACC score (C = 0.59, AIC = 759). Comparison of predictive value of prognostic scores for TTFT illustrated better performance of CLL-IPI (C = 0.72, AIC = 726) followed by Barcelona–Brno (C = 0.68, AIC = 743), modified GCLLSG (C = 0.66, AIC = 744), and O-CLL1 index (C = 0.55, AIC = 773). The results suggest better performance of CLL-IPI in terms of both OS and TTFT as compared to other available scores in our cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tremendous heterogeneity has been observed in clinical course of patients with chronic lymphocytic leukemia (CLL) and a number of genetic, molecular, biochemical, and clinical prognostic markers have been described that may aid the clinician in defining prognosis of CLL patients. The major challenge in clinical practice is to identify a comprehensive panel of prognostic parameters from the vast array of prognostic markers that best defines the prognosis and is widely available in majority of clinical laboratories worldwide.
Although limited but considerable effort in this field has led to construction of multiple prognostic algorithms for CLL. Initially, M. D. Anderson Cancer Center (MDACC) developed a prognostic index based on six independent prognostic variables, i.e., age, serum β-2-microglobulin (β2M), absolute lymphocyte count (ALC), gender, Rai stage, and the presence of lymphadenopathy [1]. Subsequently, German CLL Study Group (GCLLSG) proposed GCLLSG index for prognostication in CLL based on age, gender, Eastern Cooperative Oncology Group (ECOG) performance status, immunoglobulin heavy chain variable region (IGHV) gene mutational status, del(17p), del(11q), serum β2M, and serum thymidine kinase (TK) levels [2]. Since serum TK, one of the major component of the GCLLSG index, is not routinely investigated at all centers, implementation of this score became challenging [3]. Molica et al. later modified the GCLLSG index [2] by excluding serum TK levels and compared it with MDACC score [1]. The comparative assessment confirmed the prognostic ability of modified GCLLSG index as well as its superiority over MDACC score for TTFT [4]. However, on excluding serum TK from the GCLLSG index, the prognostic association of the model for OS was lost [2]. Later, Gentile et al. demonstrated prognostic utility of an index based on Rai stage, β2M, ALC, and IGHV mutation status in terms of TTFT [5].
With the availability of so many scores, dissimilar applicability in different populations and simultaneous emergence of newer prognostic markers, the prognostic ability of 27 well-established prognostic markers was evaluated by the international consortium on CLL in a cohort of 3472 patients enrolled in phase 3 trials from France, Germany, Poland, UK, and USA. Based on five most significant parameters, i.e., clinical Rai stage, age, β2M, IGHV mutational status and TP53 deletion and/or mutation, the international consortium on CLL proposed a comprehensive international prognostic index (CLL-IPI; International CLL-IPI working group, 2016) [6]. CLL-IPI was initially designed for prediction of OS, but later validation studies extended its utility in prediction of TTFT, as well in early-stage CLL patients [7, 8]. Delgado et al. recently proposed a simplified version of CLL-IPI, i.e., Barcelona–Brno index comprising of only two biomarkers: IGHV mutation status and FISH cytogenetics [9]. However, a comparison between CLL-IPI and the Barcelona–Brno prognostic indices on an Italian–American cohort of patients suggested better prognostic ability of CLL-IPI for progression as well as survival [10].
The ethnic differences and population-based variability and the populations on which these scores have been developed stipulate validation of the CLL-IPI prior to its broader implementation on Indian population. The present study was thus designed to evaluate the applicability and reproducibility of this score in Indian CLL cohort.
Materials and methods
Patients
A total of 198 treatment-naive CLL patients were evaluated to generate the prognostic scores. The diagnosis was established as per the International Workshop on CLL (IWCLL) criteria for diagnosis of CLL [11]. The informed consent was obtained from all the participants as per the guidelines of Institute’s Ethics Committee.
Clinical and laboratory parameters
Information regarding clinical and laboratory parameters including performance status, gender, age, Rai stage, number of palpable lymph node sites, and ALC were obtained from medical records of the patients. Serum β2M levels were estimated using human ELISA kits (R&D systems, Minneapolis, MN, USA) with assay range (0–12.5 μg/mL). The IGHV gene mutation status was determined using c-DNA as outlined previously [12].
Copy number variations
Copy number variations at loci frequently aberrant in CLL were investigated using multiplex ligation-dependent probe amplification (MLPA). The P040 CLL probe mix consisting of probes against the genomic regions 17p13, the RB1/DLEU/MIR15A-16 region on 13q14, 11q22 (ATM gene), and chromosome 12 was used. Samples were processed as per the manufacturer’s instructions (MRC Holland, The Netherlands) and data was interpreted using the Coffalyser.Net software (MRC Holland, The Netherlands). Relation dosage quotient values < 0.8 were used to determine deletion of TP53 region at 17p13, the RB1/DLEU/MIR15A-16 region on 13q14 and 11q22 (ATM gene), and values > 1.2 indicated amplification of chromosome 12 in patient samples.
Prognostic index scoring
Using the weighted grades assigned to involved variables as per different prognostic indices, scores were generated for each patient n = 198. The prognostic ability of CLL-IPI for TTFT and OS was compared with other available indices.
Survival analysis
TTFT was defined as the time from the date of diagnosis to date of commencement of first therapy, and OS was defined as the time from the date of diagnosis to date of death or date of last follow-up. The Kaplan–Meier method of survival analysis including the log rank test was used for estimations and comparisons of TTFT and OS. Hazard ratios (HRs) and confidence intervals (CIs) for HR were calculated according to the Cox proportional hazard model. The predictive power of prognostic models was assessed using the Harrell’s C-index where a value ≥ 0.7 was considered significant, and the Akaike’s information criterion (AIC), where lower AIC values indicated higher prognostic accuracy of the predictive model. All the statistical tests were carried out using the STATA/SE software ver 14.2 (StataCorp LP, College Station, TX, USA), and results were considered statistically significant at p ≤ 0.05.
Results
Baseline clinical and laboratory characteristics of patients, IGHV mutational status, and copy number variations at various loci are listed in Table 1. During the follow-up period, 138/198 (70%) required initiation of therapy of which 62 (45%) received chlorambucil-based therapy, 56 (41%) received rituximab-based therapy, and 20 (14%) received other therapies. As per Rai staging criteria [13], 71% patients were categorized as early-stage CLL (Rai 0–2), and 89 (63.5%) of these required initiation of therapy during the course of follow-up. The median follow-up time was 40.5 months (range, 1–215 months). During the study period, 86 patients died of which 78 died due to disease progression.
Patients were risk stratified as per CLL-IPI, Barcelona–Brno index, O-CLL1 score, modified GCLLSG index, and MDACC score (Table 2). On univariate analysis of nine parameters involved in designing of various scores including gender, lymph node groups, ALC, ECOG PS, age, Rai stage, copy number variations, β2M, and IGHV mutational status, all parameters except gender and age were statistically significantly associated with OS (n = 198; gender: p = 0.38, age: p = 0.8; Supplementary Table 1 and Supplementary Fig. 1) and TTFT (n = 140; gender: p = 0.72, age: p = 0.8; Supplementary Table 1 and Supplementary Fig. 2). Upon subjecting parameters with p ≤ 0.05 to multivariate analysis, Rai stage, del(17p), and IGHV mutational status retained independent prognostic association with OS, and Rai stage 2, del(17p), IGHV mutational status, performance status, and β2M retained independent prognostic association with TTFT.
On stratifying the patients according to CLL-IPI score, significant differences were observed in OS and the median OS was 28, 62, and 190 months and not reached for very high-, high-, intermediate-, and low-risk groups, respectively (p < 0.001; Fig. 1a). The multiple pairwise comparison demonstrated significant differences in all the pairs except high vs. intermediate group. As compared to low-risk group, the HR for death for intermediate risk was 5.5 (95% CI = 1.25–24.21, p = 0.024), for high risk was 8.7 (95% CI = 2.09–36.3, p = 0.003) and for very high risk group was 17.2 (95% CI = 3.9–74.7, p < 0.001). The comparisons for OS for other scores are shown in Fig. 1 and Supplementary Table 2.
Since the major challenge faced by the clinicians is management of early-stage CLL patients, we analyzed the TTFT for early stage (Rai stage 0–2, n = 140) CLL patients. Comparison of median TTFT among the four risk groups in CLL-IPI revealed a significant association between TTFT and risk groups (p < 0.001). Median TTFT for very high-, high-, and intermediate-risk groups was 1, 9, and 51 months, respectively, while for low-risk group, median TTFT could not be reached (Fig. 2a). The COX PH model suggested that as compared to low-risk group, the HR for treatment for intermediate risk was 4.1 (95% CI = 1.39–12.04, p = 0.010), for high risk was 9.7 (95% CI = 3.45–27.3, p < 0.001), and for very high risk group was 25.10 (95% CI = 8.06–78.13, p < 0.001).The TTFT for other risk categories of scores compared is shown in Fig. 2 and supplementary Table2.
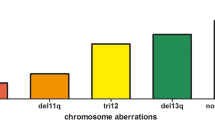
The TTFT and OS were also compared in patient groups with copy number variations at 17p13, 13q14, 11q22, and chromosome 12. As compared to patients with no genomic aberrations, median OS was significantly shorter for patients harboring del(17p) (HR 2.12, 95% CI 1.20–3.76, p = 0.009; Fig. 3a), and median TTFT was significantly lower for patients harboring del(17p) (HR 2.25, 95% CI 1.28–3.9, p = 0.005) and del(11q) (HR 2.13, 95% CI 1.05–4.3, p = 0.03; Fig. 3b).
The prognostic value of CLL-IPI was then compared with MDACC and Barcelona–Brno score for OS (Table 3). Harrell’s C-value of 0.70, necessary threshold to have a model prognostic value at individual patient level, could not be reached for any of the model for OS. Harell’s C-value was highest for CLL-IPI (C = 0.64) followed by Barcelona–Brno index (C = 0.61) and MDACC score (C = 0.59). The AIC values were lowest for CLL-IPI (AIC = 740), followed by Barcelona–Brno index (AIC = 754) and MDACC score (AIC = 759).
The prognostic value of CLL-IPI was then compared with Barcelona–Brno score, modified GCLLSG score and O-CLL1 score for TTFT (Table 3). Harrell’s C-value was highest for CLL-IPI (C = 0.72), followed by Barcelona–Brno (C = 0.68), modified GCLLSG (C = 0.66), and O-CLL1 index (C = 0.53). The AIC values also followed the same trend, and the AIC was lowest for CLL IPI (AIC = 726) as compared to Barcelona–Brno (AIC = 743), modified GCLLSG (AIC = 751), and O-CLL1 index (AIC = 773).
Discussion
Since CLL is largely heterogeneous disease, prognostic assessment of patients is essential from clinical perspective. The initial MDACC prognostic index designed to predict OS in CLL patients was validated by several groups [1, 14,15,16]. In the last decade, a number of prognostic scores have also been proposed by other groups working in different regions of the world. The latest in the series is the score developed by the international consortium on CLL (CLL-IPI; International CLL-IPI working group, 2016) [5]. The score was initially designed in terms of OS, but later validation studies extended its utility for TTFT as well [7, 8]. The present study thus aimed to assess the reproducibility and validity of recently developed CLL-IPI in Indian CLL cohort. An additional aim of the present study was to compare the efficiency of CLL-IPI with other available prognostic scores. The data available to us for the variables associated with the existing indices restricted the comparison of CLLIPI with MDACC [1] and Barcelona–Brno score [9] for OS and Barcelona–Brno score [9], modified GCLLSG score [4], and O-CLL1 score [5] for TTFT.
Of the nine parameters involved in categorization of different risk scores, all parameters except age and gender were significantly associated with OS and TTFT in univariate analysis. Although the median age of the patients analyzed in our series was almost similar to the patients evaluated for CLL-IPI, age was not significantly associated with OS and TTFT. In multivariate analysis also, only Rai stage, del(17p) and IGHV mutational status retained independent prognostic association with OS, and del(17p), Rai stage 2, IGHV mutational status, performance status, and β2M retained independent prognostic association with TTFT. The differences in the results obtained in our series could be due to lower number of patients in our study as compared to other studies performed, and due to short follow-up, there are not enough events in our cohort.
Even if Harrell’s C-value of 0.7 could not be reached, the risk groups categorized according to CLL-IPI have distinct and significantly different overall survival. The AIC values obtained for the scores compared for OS in the present study suggest better performance of CLL-IPI.
The prognostic scores were further compared using TTFT as end point which is more suitable than OS for patients with early CLL as it is not affected by competing risks of death due to unrelated conditions, relapses, and impact of new therapies. In a country like India, several patients opt for conventional therapies due to financial constraint. This is clearly evident in present study as well as previous study by our group [17] where 45% and 68% patients, respectively, received chlorambucil-based therapy as first-line treatment. Significant Harrell’s C-value and lower AIC validate robust discriminatory value of CLL-IPI score for Indian CLL patients for TTFT too. Delgado et al. evaluated all possible combinations of five variables involved in CLL-IPI to identify the simplest model with minimum number of variables and developed a prognostic model based on IGHV mutational status + FISH [del(17p) and/or del(11q)] which was further validated on Barcelona–Brno patient series. However, comparison of Barcelona–Brno score with CLL-IPI demonstrated the superiority of the CLL-IPI score [10]. The present results also suggest CLL-IPI to be better than Barcelona–Brno index. The results from the present study demonstrated that among all the scores evaluated, CLL-IPI possess highest discriminatory value for TTFT in early-stage CLL patients. A comparative study by Molica et al., however, revealed better performance of O-CLL1 score as compared to CLL-IPI and GCLLSG scores for TTFT [18]. Low performance of O-CLL1 score in the present study could be explained by exclusion of del(17p) status from O-CLL1 score, which retained independent prognostic value and high risk in multivariate analysis for Indian population. The present results thus support the notion that scores involving CNV (CLL-IPI, Barcelona–Brno, and GCLLSG) perform better than others (O-CLL1 score). Although the present study evaluated patients at the time of diagnosis, a recent meta analysis of all published studies that have used CLL-IPI has revealed applicability of CLL-IPI at different time points such as at the time of diagnosis and at the time of therapy or relapse, thus confirming its utility in CLL prognostication [19].
The present study has certain limitations. In the present study, only TP53 deletions were used to document TP53 aberrations because of unavailability of TP53 mutation status for all the patients. As TP53 mutation status using Sanger sequencing or next-generation sequencing is labor intensive and costly affair, the results of the present study indicate that the modified CLL-IPI which is based on TP53 deletion instead of TP53 deletion/mutation can also be applied in settings where TP53 mutation status cannot be investigated. Unlike other studies, the CNVs in the present study were assessed by MLPA instead of FISH. Alhourni et al. have already shown that both MLPA and iFISH have comparable detection rates for genomic aberrations typically associated with CLL [20]. Since the study cohort consisted of the patients treated with chemotherapy or chemoimmunotherapy, the results of the present study may not be applicable to the patients treated with novel agents such as Bruton tyrosine kinase inhibitor, phosphoinositide 3-kinase inhibitor, and bcl2 inhibitor [21]. The introduction of these novel agents have altogether changed the therapeutics in CLL. Moreover, the present study shows moderate prognostic value of all the prognostic models which could be due to small number of patients. The results support the notion that in clinical practice, CLL patients should only be treated in presence of active disease and that prognostic models can complement but not replace clinical expertise.
In conclusion, CLL-IPI is a simplified index composed of robust and easily available prognostic markers with a better prognostic potential than other existing scores in Indian cohort. The implication of this score would lead to execution of common criteria of prognostication worldwide which will allow multicentric comparisons and collaborations.
References
Wierda WG, O'Brien S, Wang X, Faderl S, Ferrajoli A, Do KA, Cortes J, Thomas D, Garcia-Manero G, Koller C, Beran M, Giles F, Ravandi F, Lerner S, Kantarjian H, Keating M (2007) Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood 109:4679–4685
Pflug N, Bahlo J, Shanafelt TD, Eichhorst BF, Bergmann MA, Elter T, Bauer K, Malchau G, Rabe KG, Stilgenbauer S, Döhner H, Jäger U, Eckart MJ, Hopfinger G, Busch R, Fink AM, Wendtner CM, Fischer K, Kay NE, Hallek M (2014) Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood 124:49–62
Tam CS, Seymour JF (2014) A new prognostic score for CLL. Blood 124:1–2
Molica S, Giannarelli D, Mirabelli R, Levato L, Russo A, Linardi M, Gentile M, Morabito F (2016) Unavailability of thymidine kinase does not preclude the use of German comprehensive prognostic index: results of an external validation analysis in early chronic lymphocytic leukemia and comparison with MD Anderson Cancer Center model. Eur J Haematol 96:72–77
Gentile M, Shanafelt TD, Cutrona G, Molica S, Tripepi G, Alvarez I, Mauro FR, Di Renzo N, Di Raimondo F, Vincelli I, Todoerti K, Matis S, Musolino C, Fabris S, Vigna E, Levato L, Zupo S, Angrilli F, Consoli U, Festini G, Longo G, Cortelezzi A, Arcari A, Federico M, Mannina D, Recchia AG, Neri A, Kay NE, Ferrarini M, Morabito F (2016) A progression-risk score to predict treatment-free survival for early stage chronic lymphocytic leukemia patients. Leukemia 30:1440–1443
International CLL-IPI working group (2016) An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol 17:779–790
Molica S, Shanafelt TD, Giannarelli D, Gentile M, Mirabelli R, Cutrona G, Levato L, Di Renzo N, Di Raimondo F, Musolino C, Angrilli F, Famà A, Recchia AG, Chaffee KG, Neri A, Kay NE, Ferrarini M, Morabito F (2016) The chronic lymphocytic leukemia international prognostic index predicts time to first treatment in early CLL: independent validation in a prospective cohort of early stage patients. Am J Hematol 91:1090–1095
Gentile M, Shanafelt TD, Rossi D, Laurenti L, Mauro FR, Molica S, Cutrona G, Uccello G, Campanelli M, Vigna E, Tripepi G, Chaffee KG, Parikh SA, Bossio S, Recchia AG, Innocenti I, Pasquale R, Neri A, Ferrarini M, Gaidano G, Foà R, Morabito F (2016) Validation of the CLLIPI and comparison with the MDACC prognostic index: analysis of 1364 newly diagnosed patients. Blood 128:2093–2095
Delgado J, Doubek M, Baumann T, Kotaskova J, Molica S, Mozas P, Rivas-Delgado A, Morabito F, Pospisilova S, Montserrat E (2017) Chronic lymphocytic leukemia: a prognostic model comprising only two biomarkers (IGHV mutational status and FISH cytogenetics) separates patients with different outcome and simplifies the CLL-IPI. Am J Hematol 92:375–380
Gentile M, Shanafelt TD, Mauro FR, Laurenti L, Rossi D, Molica S, Vincelli I, Cutrona G, Uccello G, Pepe S, Vigna E, Tripepi G, Chaffee KG, Parikh SA, Bossio S, Recchia AG, Innocenti I, Pasquale R, Neri A, Ferrarini M, Gaidano G, Foà R, Morabito F (2018) Comparison between the CLL-IPI and the Barcelona-Brno prognostic model: analysis of 1299 newly diagnosed cases. Am J Hematol. 93:E35–E37
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ (2008) International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 111:5446–5456
Rani L, Mathur N, Gogia A, Vishnubhatla S, Kumar L, Sharma A, Dube D, Kaur P, Gupta R (2016) Immunoglobulin heavy chain variable region gene repertoire and B-cell receptor stereotypes in Indian patients with chronic lymphocytic leukemia. Leuk Lymphoma. 57:2389–2400
Rai K (1987) A critical analysis of staging in CLL. In: Gale R, Rai K (eds) Chronic lymphocytic leukemia: recent progress and future direction. Alan R Liss, New York (NY), p 253
Shanafelt TD, Jenkins G, Call TG, Zent CS, Slager S, Bowen DA, Schwager S, Hanson CA, Jelinek DF, Kay NE (2009) Validation of a new prognostic index for patients with chronic lymphocytic leukemia. Cancer 115:363–372
Bulian P, Tarnani M, Rossi D, Forconi F, Del Poeta G, Bertoni F, Zucca E, Montillo M, Pozzato G, Deaglio S, D'Arena G, Efremov D, Marasca R, Lauria F, Gattei V, Gaidano G, Laurenti L (2011) Multicentre validation of a prognostic index for overall survival in chronic lymphocytic leukaemia. Hematol Oncol 29:91–99
Gentile M, Mauro FR, Rossi D, Vincelli I, Tripepi G, Recchia AG, De Stefano L, Campanelli M, Giannarelli D, Bossio S, Morabito L, Vigna E, Gaidano G, Foà R, Morabito F (2014) Italian external and multicentric validation of the MD Anderson Cancer Center nomogram and prognostic index for chronic lymphocytic leukaemia patients: analysis of 1502 cases. Br J Haematol 167:224–232
Gogia A, Sharma A, Raina V, Kumar L, Vishnubhatla S, Gupta R, Kumar R (2012) Assessment of 285 cases of chronic lymphocytic leukemia seen at single large tertiary center in Northern India. Leuk Lymphoma 53:1961–1965
Molica S, Giannarelli D, Levato L, Mirabelli R, Gentile M, Morabito F (2017) Assessing time to first treatment in early chronic lymphocytic leukemia (CLL): a comparative performance analysis of five prognostic models with inclusion of CLL-international prognostic index (CLL-IPI). Leuk Lymphoma 58:1736–1739
Molica S, Giannarelli D, Mirabelli R, Levato L, Kay N, Shanafelt TD (2018) Chronic lymphocytic leukemia international prognostic index: a systematic review and meta-analysis. Blood 131:365–368
Alhourani E, Rincic M, Othman MA, Pohle B, Schlie C, Glaser A, Liehr T (2014) Comprehensive chronic lymphocytic leukemia diagnostics by combined multiplex ligation dependent probe amplification (MLPA) and interphase fluorescence in situ hybridization (iFISH). Mol Cytogenet 7:79
Lamanna N, O’Brien S (2016) Novel agents in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program 2016:137–145
Funding
The financial support was provided by the Department of Biotechnology (BT/PR11106/GBD/27/145/2008, BT/PR15438/MED/30/606/2011 and BT/PR8680/AGR/36/754/2013), Ministry of Science & Technology, GOI, and All India Institute of Medical Sciences, New Delhi (8-60/A060/ 2011/RS) to RG for carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was carried out according to the Helsinki declaration. The informed consent was obtained from all the participants included in this study as per the guidelines of Ethics Committee of All India Institute of Medical Sciences.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rani, L., Gogia, A., Singh, V. et al. Comparative assessment of prognostic models in chronic lymphocytic leukemia: evaluation in Indian cohort. Ann Hematol 98, 437–443 (2019). https://doi.org/10.1007/s00277-018-3525-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3525-0