Abstract
The FIP1L1-PDGFRA (FP) fusion gene is identified in a substantial proportion of patients with eosinophilia-associated myeloproliferative neoplasms (MPN-eo) who subsequently achieve rapid and durable remissions on imatinib. In the initial diagnostic work-up of hypereosinophilia (HE), histologic and immunohistochemical evaluation of a bone marrow (BM) core biopsy is considered essential for the differentiation between reactive hypereosinophilia (HER), MPN-eo and hypereosinophilic syndrome (HES). We therefore retrospectively analysed the initial reports of BM core biopsies from 116 patients who were subsequently identified as FP positive (FP+, n = 56) or FP negative/corticosteroid-responsive HER or HES (n = 60). Compared to HER or HES, detection of FP was more frequently associated with increased numbers of blasts (11/56 vs. 2/60, p = 0.007) and mast cells (23/33 vs. 7/23, p = 0.006; with expression of CD25 [11/18 vs. 2/13, p = 0.025]), and/or fibrosis (25/35 vs. 1/23, p < 0.0001). In FP+ patients, HE was correctly associated with an underlying clonal haematologic disorder in only 36/56 (64 %) of cases, but final BM diagnoses included a variety of diagnoses such as MPN-eo (n = 15), acute myeloid leukaemia (n = 8), systemic mastocytosis (n = 6), chronic myeloid leukaemia (n = 5) or unclassified MPN (n = 2). We conclude that the final evaluation of BM core biopsies in the diagnostic work-up of HE should include comprehensive morphologic (stains for myeloid blast cells, mast cells and fibres) and genetic analyses before a final diagnosis is established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eosinophilia is commonly observed in a wide range of disparate clonal/neoplastic and non-clonal/reactive disorders. Clonal eosinophilia is usually associated with myeloid neoplasms, e.g. myeloproliferative neoplasm (MPN-eo), chronic eosinophilic leukaemia (CEL), or myelodysplastic/myeloproliferative neoplasm (MDS/MPN-eo) in chronic or blast phase and the presence of tyrosine kinase (TK) fusion genes, e.g. with involvement of PDGFRA, PDGFRB, FGFR1 or JAK2, of which the FIP1L1-PDGFRA fusion gene (FP) is by far the most frequent (FP+ MPN-eo) [1–8]. Imatinib induces rapid and durable complete clinical and haematological (CHR) remissions in almost all patients and complete molecular remissions (CMR) in >90 % of patients with a PDGFRA or PDGFRB fusion gene, conferring excellent progression free and overall survival [1, 9].
In contrast, sustained non-clonal eosinophilia with potentially life-threatening organ dysfunction, e.g. involvement of the heart, lungs, gastrointestinal tract or nervous system, is frequently associated with autoimmune disorders (reactive hypereosinophilia, HER), e.g. eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome), infections or as a diagnosis of exclusion, hypereosinophilic syndrome (HES). Irrespective of the underlying cause, patients with reactive, non-clonal eosinophilia (HER or HES) show a rapid and sustained clinical and haematological response to treatment with corticosteroid-based immunosuppression in the vast majority of patients. In cases without a final diagnosis, a rapid and durable response to corticosteroids points towards diagnosis of HER or HES and almost definitively excludes the possibility of clonal eosinophilia [2].
A bone marrow (BM) core biopsy with subsequent histology and immunohistochemistry is considered essential for the initial diagnostic work-up of unexplained hypereosinophilia (HE) and the differentiation between clonal and non-clonal eosinophilia in the absence of a cytogenetic or molecular marker. This remains, however, challenging because the current WHO 2008 classification does not include clear histopathological criteria for clonal/neoplastic and non-clonal/reactive eosinophilia with the exception of increased numbers of blasts as a major criterion for CEL, not otherwise specified (CEL-NOS) [10].
We here report on a retrospective analysis within the “German Registry on Disorders of Eosinophils and Mast Cells” of the initial histopathological and immunohistochemical evaluation of BM core biopsies in patients who were subsequently diagnosed as FP+ MPN-eo (clonal eosinophilia) or corticosteroid-responsive HER or HES (non-clonal eosinophilia). Several new key findings emerge from this study including the following: (i) Immunolabelling of myeloid blast cells and mast cells as well as grading of fibrosis is revealed to be key diagnostic points, but (ii) these techniques were not consistently applied to the majority of cases, and (iii) incorrect final diagnoses were made in a substantial proportion of patients including chronic myeloid leukaemia (CML) or systemic mastocytosis (SM).
Patients and methods
Patients
Within the “German Registry on Disorders of Eosinophils and Mast Cells”, BM core biopsy reports were available from 116 patients who were subsequently identified as FP+ (n = 56; male/female ratio 55/1; median age 49 years; range 21–70) or corticosteroid-responsive HER or HES (n = 60; male/female ratio 34/26; median age 48 years, range 18–88). Thirteen FP+ MPN-eo patients initially presented in blast phase (myeloid, n = 8; lymphoid, n = 3; myeloid sarcoma, n = 2). Only clearly diagnosed HER or HES patients with direct (positive histology, MRI revealing intracardial fibrosis or thrombus) or indirect (e.g. lung infiltrates, eosinophilic effusions or bronchoalveolar lavage, splenomegaly) signs of eosinophilia-associated organ involvement/dysfunction and documented sustained clinical and haematological response to corticosteroids were enrolled. Twenty patients were not enrolled because their response to corticosteroid-based immunosuppressive treatment was absent (n = 10) or unknown (n = 10).
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Evaluation of BM core biopsies
The BM core biopsies were processed and diagnosed predominantly by practising surgical pathologists and only rarely by specialized haematopathologists at a variety of pathology laboratories all over Germany. The histopathological and immunohistochemical features, which were obtained from the initial BM biopsy pathological reports, included BM cellularity, numbers of eosinophils, blasts and mast cells, and fibrosis (Gomori’s staining). Additional immunohistological stainings included expression of CD34 (blasts) and mast cells with a mature immunophenotype (tryptase/CD117) or aberrant expression of CD25. The histomorphological diagnoses were reevaluated according to the presence or absence of FP and the response to corticosteroids. The median time from histopathological assessment to the initiation of molecular genetic analysis was 1 month (range 0–82).
FIP1L1-PDGFRA
RT-PCR for FP was performed as previously described [11].
Clinical cases
Here, we describe three clinical cases with MPN-eo illustrating the difficulties encountered in the assessment of BM histology in FP+ patients.
Case 1
A BM biopsy was performed in a 34-year-old male patient because of urticaria, arthralgia and significant eosinophilia (18 × 109/l, 81 %). The final histopathological diagnosis was SM with 15 % mast cell infiltration, and the karyotype was normal. However, the patient did not respond to antimediator treatment. At referral, 3 months later and 12 months after start of symptoms, peripheral blood (PB) was negative for KIT D816V but positive for FP. Treatment with imatinib (100 mg/d) led to rapid and sustained complete clinical, haematological (CHR) and molecular remission (CMR) after 2 months.
Case 2
In a 69-year-old male patient with eosinophilia (5.7 × 109/l; 52 %), reactive causes and the FP fusion gene were excluded by in-house molecular testing at a university hospital. Because eosinophilia persisted and the spleen size increased to 15 cm, a full diagnostic work-up was performed including CT-scan, endoscopy of the gastrointestinal tract and BM core biopsy. Final diagnosis was SM with 5 % mast cell infiltration and normal serum tryptase levels. JAK2 V617F, BCR-ABL and particularly KIT D816V were tested negative and cytogenetic analysis was not informative. Twelve months later, the patient was referred because of persisting eosinophilia. He tested FP+ by RT-PCR analysis and his BM core biopsy showed typical features of FP+ MPN-eo including elevated numbers of loosely scattered mast cells and fibrosis. He achieved rapid and sustained response on imatinib.
Case 3
A 50-year-old patient was referred because of a 6-month history of urticaria, pruritus and significant eosinophilia (5.4 × 109/l, 40 %). A BM histology revealed reactive changes without signs for a MPN, and the karyotype was normal. Eighteen months later, worsening of thrombocytopenia (100 × 109/l) and eosinophilia (60 %) became evident. A second BM histology revealed a MPN-like disease, and FP could be identified 28 months after start of symptoms and 21 months after first evidence of eosinophilia. On imatinib, the patient achieved CHR and CMR after 8 months.
Results
Peripheral blood counts
In PB, no differences were identified between FP+ MPN-eo and HER or HES regarding white blood cell counts (WBC, median 14.3 × 109/l, range 4.3–173.0, vs. 14.0 × 109/l, range 4.6–111.0; p = n.s.), absolute (6.7 × 109/μl, range 0.9–120.0, vs. 5.2 × 109/l, range 0.5–94.4; p = n.s.) and relative (46 %, range 6–82, vs. 39 %, range 1–84; p = n.s.) numbers of eosinophils and haemoglobin (12.3 g/dL, range 7.1–15.6, vs. 13.3, range 6.8–16.2; p = n.s.). Significant differences were observed regarding median platelet count (151 × 109/l, range 11–600, vs. 258 × 109/l, range 57–260; p < 0.0001) and presence of blast cells (8/56, 14 %, vs. 0/60, p = 0.002; Table 1). Serum tryptase levels were available for 23/56 (41 %) FP+ patients (median 25, range 4–183) and 33/60 (55 %) HER or HES patients (median 6.5, range 1–69).
BM morphology
BM cellularity was described in the majority of patients (FP+MPN-eo, n = 56, 100 %, vs. HER or HES, n = 53, 88 %). Hypercellularity pointed towards diagnosis of clonal eosinophilia (45/56, 80 % vs. 23/52, 43 %; p < 0.0001), whereas a hypocellular BM was only reported in a few cases of HER or HES (n = 2/60, 3 %) but never in FP+ MPN-eo. The median relative number of eosinophils was 43 % (range 5–80) vs. 26 % (range 5–80, p = n.s.; Table 2).
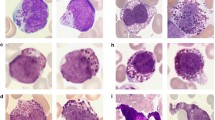
Increased numbers of CD34 positive blasts favoured diagnosis of FP+ MPN-eo (11/56, 20 %, vs. 2/60, 3 %, p = 0.007). The number and morphology of mast cells were only reported in 53/116 (46 %) of all patients. An increase of loosely scattered mast cells was clearly associated with FP+ MPN-eo (23/30, 70 %, vs. 7/23, 30 %, p = 0.006, Fig. 1). Clustering or dense infiltrates of mast cells were not seen in any patient. Immunohistochemistry for expression of tryptase/CD117 (17/17, 100 % vs. 4/12, 33 %; p < 0.05) and CD25/CD2 (11/18, 61 % vs. 2/13, 15 %; p = 0.025) was clearly in favour of FP+ MPN-eo but was only performed in a minority of patients. Gomori staining for BM fibrosis was only performed in 58/116 (50 %) of all patients, although BM fibrosis ≥I° was a characteristic feature for FP+ MPN-eo (25/35, 71 %; I°, n = 7; II°, n = 8; III°, n = 10; vs. 1/23, 4 %, p < 0.0001; Table 2).
Bone marrow biopsy in a FIP1L1-PDGFRA+ MPN-eo. a Hypercellular bone marrow biopsy with a marked infiltration by eosinophils and their precursors (Giemsa stain). b Infiltration by loosely scattered mast cells (tryptase stain; Horn Imaging Camera, Aalen, Germany; Zeiss Imager.M1, Carl Zeiss, Oberkochen, Germany)
Diagnoses according to BM morphology
In FP+ MPN-eo, clonal eosinophilia was correctly diagnosed in 36/56 (64 %) patients. Preliminary diagnoses included MPN-eo (n = 15), MPN-unclassified/acute leukaemia (n = 10), SM (n = 6) and CML (n = 5). Non-clonal eosinophilia or no final conclusion was reported in 13/56 (23 %) and 7/56 (13 %) patients, respectively. In HER or HES, non-clonal eosinophilia was diagnosed in 43/60 (72 %) patients. Clonal eosinophilia or no final conclusion was reported in 7/60 (12 %) and 10/60 (17 %) patients, respectively. In summary, the overall sensitivity for reliable diagnosis of clonal or non-clonal eosinophilia was 68 % (79/116; Table 3).
Discussion
We retrospectively analysed the diagnostic impact of the initially evaluated histopathological und immunohistochemical BM characteristics in patients with HE who were subsequently identified as FP+ MPN-eo or corticosteroid-responsive HER or HES. In addition to clinical and genetic characteristics, the rapid and durable response to corticosteroids in HER or HES was chosen as an important clinical feature to exclude the possibility of unsuspected clonal eosinophilia.
As previously reported, the presence of the FP fusion gene was strongly associated with increased numbers of loosely scattered mast cells and fibrosis [1]. In our series, mast cell staining and assessment of fibrosis were, however, only performed in half of the patients. The simultaneous presence of mast cells and fibrosis was only reported in FP+ MPN-eo (9/56, 16 %) but not in any case of HER or HES. The presence of loosely scattered mast cells can easily be missed in HE-stained BM biopsy specimen if the expression of tryptase/CD117 in typical and the coexpression of CD25 in atypical mast cells is not analysed. In our series, those immunostainings were performed in only 36 % of patients (Table 3). An increased number of myeloid blasts, which is the only morphological feature included in the WHO 2008 classification, was only found in rare cases of FP+ MPN-eo, particularly in myeloid blast phase. In our analysis, blasts were therefore no relevant feature for the differentiation between HER or HES and FP+ MPN-eo in chronic phase.
BM hypercellularity was also more frequently observed in FP+ MPN-eo, but the relative frequency of 35 % cases with hypercellularity reported in HER or HES emphasizes that it should be interpreted with caution, and only in combination with mast cells and fibrosis. The weakest morphological parameters include absolute and relative numbers of eosinophils in PB and BM, which do not allow discrimination between clonal and non-clonal eosinophilia. In this respect, it should be pointed out that the absolute number of eosinophils in PB of 56 patients with FP+ MPN-eo in chronic phase was less than 1.5 × 109/l in 5 % (3/56) of cases. Neither the arbitrary boundary of 1.5 × 109/l eosinophils nor the 6-month interval should therefore be applied in patients with TK fusion gene driven clonal eosinophilia, e.g. due to involvement of PDGFRA, PDGFRB, FGFR1 or JAK2. Although the serum tryptase levels are significantly different between FP+ MPN-eo and HER or HES, this most valid clinical parameter was only measured in a subset of patients. Based on our data and as previously published, we emphasize that the serum tryptase level should become a basic parameter in the diagnostic work-up of unexplained hyperosinophilia [12, 13].
Most likely as a consequence of the lack of validated and standardized diagnostic morphological criteria and the infrequent use of appropriate stainings, clonal eosinophilia was only suggested in 64 % (two thirds) of patients that were subsequently diagnosed as FP+ MPN-eo. Surprisingly, the initial diagnoses included CML, SM, MPN-u and AML in a substantial proportion of patients. These findings add to our recent report on patients with MPN-eo and PDGRFA or PDGFRB fusion genes in myeloid or lymphoid blast phase [1]. Eight of 17 patients were initially diagnosed and treated as “AML” or “T-cell lymphoma” with intensive chemotherapy ± allogeneic stem cell transplantation but experienced resistance and/or relapse. After diagnosis of the underlying FP fusion gene, all eight patients achieved complete remission on treatment with imatinib. The clinical consequences of cases not being investigated for the presence or absence of FP because of misleading morphological diagnosis such as CML, MPN-u, SM (cases 1 and 2), AML, T-cell lymphoma, or HER or HES (case 3) may be considerable. Two of the three patients reported in the clinical cases above suffered severely from symptoms leading to incapacity to work. Once correctly diagnosed and treated with imatinib, their conditions completely resolved. Madelung et al. [14] recently reported on a consensus between histological features and final clinical diagnosis between 70 and 82 % in classical MPN, but the therapeutic consequences of a misdiagnosis of classical MPN subtype are usually much less serious than it would be for diagnosis of FP+ MPN-eo vs. HER or HES. The diagnostic sensitivity of BM morphology was better in HER or HES than in MPN-eo although a minority of patients (12 %) was diagnosed as clonal (MPN-eo in all cases). The therapeutic consequences in this situation may also be significant, as life-saving treatment with corticosteroids may be withheld from affected patients.
In conclusion, our data highlight several new aspects regarding the role of BM examination in the diagnostic work-up of HE: (a) The absolute and relative numbers of eosinophils in BM (and PB) are not useful to differentiate between clonal and non-clonal eosinophilia; (b) the evaluation of blasts (CD34), mast cells (including CD117/tryptase/CD25) and fibrosis are important features for FP+ MPN-eo, and the respective stainings should be firmly integrated in the diagnostic work-up of HE; and (c) the final diagnosis should be addressed with caution and possibly only made with full knowledge of relevant clinical, cytogenetic and molecular data. Appropriate use of the available techniques should allow an improvement of the currently limited diagnostic value and sensitivity of routine BM examinations in the diagnostic work-up of HE.
References
Metzgeroth G, Schwaab J, Gosenca D, Fabarius A, Haferlach C, Hochhaus A, Cross NC, Hofmann WK, Reiter A (2013) Long-term follow-up of treatment with imatinib in eosinophilia-associated myeloid/lymphoid neoplasms with PDGFR rearrangements in blast phase. Leukemia 27(11):2254–2256
Valent P, Klion AD, Rosenwasser LJ, Arock M, Bochner BS, Butterfield JH, Gotlib J, Haferlach T, Hellmann A, Horny HP, Leiferman KM, Metzgeroth G, Matsumoto K, Reiter A, Roufosse F, Rothenberg ME, Simon HU, Sotlar K, Vandenberghe P, Weller PF, Gleich GJ (2012) ICON: eosinophil disorders. The World Allergy Organization journal 5(12):174–181
Walz C, Score J, Mix J, Cilloni D, Roche-Lestienne C, Yeh RF, Wiemels JL, Ottaviani E, Erben P, Hochhaus A, Baccarani M, Grimwade D, Preudhomme C, Apperley J, Martinelli G, Saglio G, Cross NC, Reiter A (2009) The molecular anatomy of the FIP1L1-PDGFRA fusion gene. Leukemia 23(2):271–278
Walz C, Cross NC, Van Etten RA, Reiter A (2008) Comparison of mutated ABL1 and JAK2 as oncogenes and drug targets in myeloproliferative disorders. Leukemia 22(7):1320–1334
Walz C, Metzgeroth G, Haferlach C, Schmitt-Graeff A, Fabarius A, Hagen V, Prummer O, Rauh S, Hehlmann R, Hochhaus A, Cross NC, Reiter A (2007) Characterization of three new imatinib-responsive fusion genes in chronic myeloproliferative disorders generated by disruption of the platelet-derived growth factor receptor beta gene. Haematologica 92(2):163–169
Walz C, Chase A, Schoch C, Weisser A, Schlegel F, Hochhaus A, Fuchs R, Schmitt-Graff A, Hehlmann R, Cross NC, Reiter A (2005) The t(8;17)(p11;q23) in the 8p11 myeloproliferative syndrome fuses MYO18A to FGFR1. Leukemia 19(6):1005–1009
Schwaab J, Knut M, Haferlach C, Metzgeroth G, Horny HP, Chase A, Tapper W, Score J, Waghorn K, Naumann N, Jawhar M, Fabarius A, Hofmann WK, Cross NC, Reiter A (2015) Limited duration of complete remission on ruxolitinib in myeloid neoplasms with PCM1-JAK2 and BCR-JAK2 fusion genes. Ann Hematol 94(2):233–238
Metzgeroth G, Walz C, Erben P, Popp H, Schmitt-Graeff A, Haferlach C, Fabarius A, Schnittger S, Grimwade D, Cross NC, Hehlmann R, Hochhaus A, Reiter A (2008) Safety and efficacy of imatinib in chronic eosinophilic leukaemia and hypereosinophilic syndrome: a phase-II study. Br J Haematol 143(5):707–715
Metzgeroth G, Erben P, Martin H, Mousset S, Teichmann M, Walz C, Klippstein T, Hochhaus A, Cross NC, Hofmann WK, Reiter A (2012) Limited clinical activity of nilotinib and sorafenib in FIP1L1-PDGFRA positive chronic eosinophilic leukemia with imatinib-resistant T674I mutation. Leukemia 26(1):162–164
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114(5):937–951
Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, Kutok J, Clark J, Galinsky I, Griffin JD, Cross NC, Tefferi A, Malone J, Alam R, Schrier SL, Schmid J, Rose M, Vandenberghe P, Verhoef G, Boogaerts M, Wlodarska I, Kantarjian H, Marynen P, Coutre SE, Stone R, Gilliland DG (2003) A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med 348(13):1201–1214
Klion AD, Noel P, Akin C, Law MA, Gilliland DG, Cools J, Metcalfe DD, Nutman TB (2003) Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood 101(12):4660–4666
Valent P, Sperr WR, Sotlar K, Reiter A, Akin C, Gotlib J, Horny HP, Arock M (2014) The serum tryptase test: an emerging robust biomarker in clinical hematology. Expert Rev Hematol 7(5):683–690
Madelung AB, Bondo H, Stamp I, Loevgreen P, Nielsen SL, Falensteen A, Knudsen H, Ehinger M, Dahl-Sorensen R, Mortensen NB, Svendsen KD, Lange T, Ralfkiaer E, Nielsen K, Hasselbalch HC, Thiele J (2013) World Health Organization-defined classification of myeloproliferative neoplasms: morphological reproducibility and clinical correlations—the Danish experience. Am J Hematol 88(12):1012–1016
Acknowledgments
This work was supported by the “Deutsche José Carreras Leukämie-Stiftung e.V.” (H11/03 and R13/05), Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Authorship and disclosure
JS, MJ, NN, AF and GM performed the data analysis. AR, GM and WKH provided patient material. ASG and HPH reviewed the bone marrow biopsies. JS, NCPC, AR and GM wrote the paper. NCPC, WKH and AR revised the manuscript.
Additional information
Andreas Reiter and Georgia Metzgeroth contributed equally to this work.
Rights and permissions
About this article
Cite this article
Schwaab, J., Jawhar, M., Naumann, N. et al. Diagnostic challenges in the work up of hypereosinophilia: pitfalls in bone marrow core biopsy interpretation. Ann Hematol 95, 557–562 (2016). https://doi.org/10.1007/s00277-016-2598-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-016-2598-x