Abstract
Myelodysplastic syndrome (MDS) is a clonal hematopoietic stem cell disorder characterized by dysplastic changes in the bone marrow, ineffective erythropoiesis, and an increased risk of developing acute myeloid leukemia. Treatment planning for patients with MDS is a complex process, and we sought to better characterize hematopoietic cell transplantation (HCT) outcomes and the factors that play into decision-making regarding referral of adults with MDS for definitive therapy with HCT. Patients enrolled in a population-based study of MDS between April 2010 and January 2013 who underwent HCT within the first year after enrollment were included in this analysis. Age- and risk-matched MDS patient controls also enrolled during that time period were used as a comparison. Survival was significantly better in the HCT group (48 vs. 21 %, log-rank p value 0.009). Non-HCT patients were more likely to have comorbidities, and HCT patients were more likely to have a college degree and an income >$80,000. All three of these variables were independently associated with HCT, but none impacted survival. Patients with MDS in our study who underwent HCT had better survival than a comparable group of patients who did not undergo HCT. With refined treatment techniques, more patients may be able to be considered for this therapy. More work needs to be done to determine why education and income appear to impact the decision to pursue HCT, but these factors may impact referral to an academic center where aggressive therapy like HCT is more likely to be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myelodysplastic syndrome (MDS) is a clonal hematopoietic stem cell disorder characterized by dysplastic changes in the bone marrow, ineffective erythropoiesis, and an increased risk of developing acute myeloid leukemia [1, 2]. MDS is generally a disease of the elderly with a median age at diagnosis of 65–70 years [3]. MDS occurs in 3–4 individuals per 100,000 in the US population [4], although recent evidence suggests that the incidence is much higher [5]. The prevalence of MDS increases with age and males are more commonly affected than females [4]. MDS is a heterogeneous disorder and classification schemes have evolved over the years. An updated World Health Organization (WHO) system was recently developed to more clearly define pathologic criteria [6]. Prognosis of MDS varies greatly depending on a multitude of patient- and disease-specific factors. Much work has been done in the development of scoring systems that aid prognostication and treatment planning. The most widely used risk stratification systems include the International Prognostic Scoring System (IPSS), the Revised IPSS (IPSS-R), and the WHO Prognostic Scoring System (WPSS) [7–10].
Treatment strategies for MDS have evolved over the years. Supportive care measures, including transfusions and growth factors, can be used to ameliorate symptoms of cytopenias in low-risk MDS while aggressive chemotherapy is used to treat more advanced MDS, but hematopoietic cell transplantation (HCT) is the only curative option. Recently, hypomethylating agents including decitabine and azacytidine have been increasingly used. These agents can alter the disease course, improve survival, and in some cases serve as a bridge to HCT [11–15]. Choice of a treatment strategy depends on a number of factors, but most employ a risk-based treatment approach based on the IPSS risk stratification. Survival in patients with untreated intermediate 2 or high-risk disease is generally less than a year, so modifying the disease course and avoiding progression to acute leukemia are often the goals. Aggressive treatment including hypomethylating agents and HCT are considered in these patients. In low- or intermediate-1-risk disease, survival is longer so a less aggressive approach to maintain quality of life is generally utilized [2, 3]. Given that MDS is most often a disease of the elderly, other factors that need to be taken into consideration include age at diagnosis, functional status, and comorbidities. Other factors may play into decision-making as well but are not easy to discern or quantify.
Deciding on a treatment plan for patients with MDS is a complex process. With improved outcomes after HCT and the increased utilization of reduced-intensity conditioning regimens, we sought to better characterize the factors that play into decision-making regarding referral of adults with MDS for definitive therapy with HCT by reporting the outcomes of patients enrolled in a Minnesota population-based study who have undergone HCT and comparing them with adults with MDS who did not receive HCT and who are of similar age and disease risk.
Materials and methods
All procedures followed were in accordance with the ethical standards of the institutional review board and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients included in the study.
Study population and design
Adults in Minnesota with Myelodysplastic Syndromes (AIMMS) is a statewide prospective population-based study conducted by the University of Minnesota (UMN), Mayo Clinic, and Minnesota Department of Health. In April 2010, the Minnesota Cancer Surveillance System (MCSS) began rapid case ascertainment of all newly diagnosed adult cases (ages 20+ years) of MDS. Following physician approval, patients were contacted for invitation to enroll. Sixty-five percent of identified cases have been enrolled. An extensive questionnaire to gather epidemiologic data was completed by each participant at study entry. Central medical review, consisting of independent pathology review of bone marrow and peripheral blood samples by two hematopathologists along with independent cytogenetic interpretation by a cytogeneticist, was then performed starting at MDS diagnosis. Reports were integrated, with discrepancies in WHO classification requiring collaboration for a unifying subtype according to the 2008 revised criteria [6]. Cases without diagnostic verification were excluded.
The pathologic review was coupled with a chart review by an oncologist to assign prognostic risk scores (IPSS and/or IPSS-R) [7, 8]. Scores were calculated independently for each patient, and lack of necessary cytogenetic and hematologic data to calculate risk scores resulted in case exclusion. Treatment exposures and responses were abstracted, including relevant exposures prior to formal diagnosis when available. Per the AIMMS study design, enrollment of cases diagnosed before November 2014 will continue into early 2015, with annual clinical review of all patient cases planned for 3 years following their initial enrollment.
Patients enrolled on AIMMS between April 2010 and January 2013 who had at least 1 year of prospective follow-up were included in this analysis, which consisted of two parts. The first was a description of characteristics and outcomes of patients enrolled during this time period who underwent HCT. The second was a comparison of the patients who underwent HCT to age- and risk-matched MDS controls enrolled on AIMMS during the same time period who did not undergo HCT. Additional medical record review for all patients in this analysis included consideration of HCT, comorbidities, HCT-specific data, and HCT outcomes. Academic centers were defined as the UMN and Mayo Clinic, with all other sites designated as community-based practices. Comorbidities were gathered from the questionnaire and medical records. In an attempt to categorize them with regard to severity, we utilized the HCT-CI weighing and scoring system. A score of 0 was considered low risk, 1–2 intermediate risk, and 3+ high risk [16].
Statistical methods
Contingency table methods were used for comparison of categorical data. Kaplan-Meier survival curves were used to compare differences in survival between HCT and non-HCT patients. We used Cox proportional hazards regression to compute crude and adjusted hazard ratios (HR) and 95 % confidence intervals (CI) to identify predictors of survival among cases who underwent HCT and between HCT and non-HCT cases. Age at diagnosis (continuous), sex, WHO MDS classification (RAEB-1, RAEB-2, RCMD, tMDS, other), IPSS-R (low or intermediate, high, very high), WPSS (very low/low/intermediate, high, very high), number of comorbidities (0–1, 2–3, 4+), comorbidity risk score (low risk, intermediate risk, high risk), education (≤high school, some post high school, college graduate), and income (≤$40,000, $40,000–80,000, >$80,000) were evaluated as potential confounders. Variables were included in the final model if they changed the magnitude of the HR for HCT > 10 %. All statistical analyses were conducted using SAS (Version 9.3, Cary, NC) and all reported p values are two-sided.
Results
Patient characteristics
A total of 342 patients were enrolled on AIMMS between April 2010 and January 2013. Of those, 33 (9.6 %) underwent HCT in the first year after enrollment. Thirty-two patients of similar age and disease risk enrolled during the same time period and not undergoing HCT were identified to serve as a comparison to the HCT patients. In these control patients, the reasons for not pursuing HCT included comorbidities (n = 8), progressive disease (n = 8), patient refusal (n = 3), financial reasons and lack of a caregiver (n = 1), and no consideration of HCT documented (n = 12). Characteristics of the 65 patients analyzed are outlined in Table 1. The median age at diagnosis of MDS was 59 years (range 25–70) in the HCT group and 63 years (range 51–75) in the non-HCT group (p = 0.07). There were no significant differences in MDS severity between the HCT and non-HCT groups based on WHO classification, IPSS-R risk category, or WPSS risk category. Sex, marital status, and ethnicity were similar. The ethnic background of the majority of patients (non-Hispanic white) reflects the population in Minnesota and those most at risk of MDS.
HCT characteristics and outcomes
The cited reasons for pursuing HCT were disease risk and age. The median time from diagnosis to HCT was 199 days (range 36–778). Most patients received some active therapy prior to HCT (standard cytotoxic chemotherapy only [n = 11], hypomethylating agent only [n = 5], hypomethylating agent and cytotoxic chemotherapy [n = 13]). Nineteen patients received their HCT at the UMN and 14 at the Mayo Clinic. Donor source was unrelated in 61 %. Graft source was peripheral blood stem cells in 61 %, double umbilical cord blood in 33 %, and bone marrow in 6 %. The majority received reduced-intensity conditioning (85 %) and cyclosporine-based graft versus host disease (GVHD) prophylaxis (72 %).
Thirty-two patients achieved neutrophil engraftment at a median of 14 days (range 6–25). GVHD occurred in 36 % of patients. The most common HCT-related complication was infection (viral, bacterial, or fungal). Median follow-up is 732 days (range 25–1506). Relapse occurred in 24 % of patients and overall survival was 48 %. Though not significant, IPSS-R risk classification appeared to predict survival among HCT patients (Table 2). Those patients with high or very high risk classification had a twofold (HR 2.34, CI 0.47–11.8, p = 0.30) and threefold (HR 3.30, CI 0.84–12.9, p = 0.09) increased risk of death, respectively. Importantly, the comorbidity score did not negatively impact survival. Sex, donor source (unrelated vs. sibling), graft source (bone marrow vs. peripheral blood stem cells vs. double umbilical cord blood), conditioning intensity, and time to engraftment also did not impact survival.
Comparison of HCT and non-HCT patients
Table 3 shows a comparison between the HCT and non-HCT groups. As noted previously, there was no significant difference in MDS risk classification between the two groups (see Table 1). Other disease characteristics were also similar between the groups. Location of disease diagnosis (urban vs. rural) was similar between the two groups, but there were more patients in the non-HCT group being treated exclusively in a community setting (38 vs. 6.1 %, p = 0.008). More non-HCT patients received supportive care only (41 vs. 9.1 %, p = 0.007). Non-HCT patients had a higher number of comorbidities (p = 0.0005) and were more likely to have a high-risk comorbidity score (p < 0.0001). HCT patients were more likely to have a college degree (61 vs. 26 %, p = 0.02) and to have an income >$80,000 (44 vs. 13 %, p = 0.02). There were no statistically significant associations between comorbidities and education level or income. When the number of comorbidities was included in an adjusted model with education and income, none of the variables was significantly associated with transplant although confidence intervals were wide. Cases with higher numbers of comorbidities were less likely to receive HCT (OR 0.23, 95 % CI 0.04–1.39 for 4+ comorbidities vs. 0–1 comorbidity), and those with higher education (OR = 3.99, 95 % CI 0.40–40 for college graduate vs. less than college graduate) and income (OR = 1.72, 95 % CI 0.25–12 for >$80,000 vs. ≤$40,000) were more likely to receive HCT.
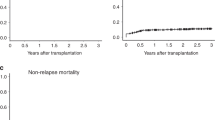
Median time from diagnosis to death or last follow-up was 847 days (211–1628) in the HCT group and 398 days (range 92–1826) in the non-HCT group. Survival was significantly better in the HCT group (48 vs. 21 %, log-rank p value 0.009) (Fig. 1). The causes of death in the HCT group include recurrent MDS (n = 8), infection (n = 3), GVHD (n = 2), post transplant lymphoproliferative disease (n = 1), and recurrent osteosarcoma (n = 1). The causes of death in the non-HCT group include progressive MDS (n = 16), infection (n = 2), pulmonary fibrosis (n = 1), pulmonary hemorrhage (n = 1), and unknown (n = 2). In a univariate model, undergoing HCT had a positive impact on survival (HR 0.43, 95 % CI 0.22–0.83, p = 0.01), while IPSS-R high (HR 6.06, 95 % CI 1.95–19, p = 0.002) and very high risk (HR 8.75, 95 % CI 2.94–26.0, p < 0.0001) classification had a negative impact on survival. The adjusted model showed similar outcomes with regard to HCT (HR 0.25, 95 % CI 0.09–0.75, p = 0.01) and IPSS-R risk classification (IPSS-R high HR 5.99, 95 % CI 1.51–23.7, p = 0.01; IPSS-R very high HR 11.1, 95 % CI 3.10–39.8, p = 0.0002). Importantly, comorbidities did not appear to negatively impact survival in our cohort of patients. Age at diagnosis, education and income group also did not appear to impact survival (Table 4).
Discussion
Determining a treatment plan for patients with MDS is a complex process, and this analysis sought to investigate differences between patients who underwent HCT and those who did not. Age, disease risk, and performance status are historically the most important factors taken into consideration when determining a treatment plan. This case-control analysis revealed several other factors, some expected and some unexpected, that also appear to play into the decision of whether to offer curative therapy with HCT. Not surprisingly, the presence and severity of comorbidities impacted whether a patient received HCT. Interestingly though, neither number nor severity of comorbidities negatively impacted survival in multivariate analysis. Unexpectedly, education and income also seemed to play a role. Patients who underwent HCT had more education and a higher income than those who did not undergo HCT though again neither of these factors impacted overall survival.
When determining whether HCT is appropriate to offer patients with MDS, many factors are taken into consideration. Age, disease risk, and comorbidities are all easily explainable, but why education and income appear to play a role is not as clear. Comorbidities are often linked with socioeconomic status, which then could explain why less education and a lower income are associated with not receiving HCT. However, in our cohort of patients, comorbidities, education, and income were all independently predictive of HCT in a multivariate model. Race, which can be associated with education and income and can also be a factor in HCT decisions since unrelated donors are less readily available in minorities, was also examined and was not different between the groups.
A recent analysis from our population-based study of MDS showed that younger patients and those treated at academic centers received more aggressive treatment approaches, a trend that was consistent across all IPSS and IPSS-R risk categories [17]. Given this information, one hypothesis to potentially explain our finding that education and income impacts the decision to pursue HCT is that education and income affect referral to an academic center and subsequently the aggressiveness of treatment. While difficult to determine in our small cohort, we performed an association analysis which revealed that those with less education appeared to receive their therapy at a community center more often than those with more education. A similar association between treatment type and income and education was seen in the larger, unselected dataset used in the analysis by Pease et al., making unintentional selection bias due to small numbers less likely.
Referral to an academic center where HCT is performed is a critical step in the treatment decision-making process. If education and income impact the likelihood of referral to an academic center and those who are treated at an academic center are more likely to receive aggressive treatment approaches, then it makes sense that those with less education and income are less likely to undergo curative therapy with HCT. It is impossible for us to determine why referrals occurred less often in these patients in our cohort, but potential reasons include inadequate insurance coverage and a lack of resources (e.g., ability to get time off work needed to undergo HCT).
Outcomes in our patients who underwent HCT are similar to other published reports showing an overall survival of 30–50 % [3, 18, 19]. A recent Center for International Blood and Marrow Transplant Research (CIBMTR) analysis of MDS patients undergoing HCT at US transplant centers revealed a 2-year overall survival of 47 and 44 % for transplants occurring between 2008–2010 and 2004–2007, respectively. This is a significant improvement when compared to patients transplanted prior to 2000 when the 2-year overall survival was 34–39 % [20]. Several factors are likely responsible for this improvement including better human leukocyte antigen matching and better management of HCT-related complications [21]. The only predictor of survival in HCT patients in our study appeared to be IPSS-R risk classification. This is not surprising as it is well described that patients with higher risk disease do less well with any therapy, including HCT [22, 23]. Even though the presence and severity of comorbidities affected whether or not HCT was offered to patients in this cohort, in contrast to other published reports [24, 25], comorbidities did not negatively impact survival. It is likely that we were unable to completely assess comorbidities in our cohort leading to an inaccurate assessment of their impact on survival. Notably, survival in our cohort is significantly better in patients who underwent HCT despite similar age and disease risk (48 vs. 21 %). This positive impact on survival remains even in multivariate analysis. Longer follow-up is needed to determine if this survival advantage persists.
Our analysis is unique in that it reports data from a population-based study of MDS that employs a rigorous central pathologic review process and direct chart review providing a high degree of diagnostic certainty, but it is limited by small numbers. In addition, despite the direct chart review process, we were only able to account for comorbidities and factors that were overtly written in the available medical records and those that were reported by the patient in their initial questionnaire. This reporting and recall bias likely results in an underestimate of the incidence and severity of comorbidities in the patient population, but should affect both the HCT and non-HCT groups equally.
In our study period, 65 % of those diagnosed with MDS in Minnesota were captured in AIMMS and 56 % had complete pathology review. Reasons for not enrolling include the following: patient deceased before contact (n = 83), language ineligible (n = 2), lost to follow-up (n = 16), refusal (n = 70), and non-participating site (n = 1). Forty-three patients are still in process for study enrollment.
Patients with MDS in our study who underwent HCT had better survival than a comparable group of patients who did not undergo HCT. With refined treatment techniques, including hypomethylating agents and reduced-intensity conditioning, more patients may be able to be considered for this therapy. More work needs to be done to determine why education and income appear to impact the decision to pursue HCT, but these factors may impact referral to an academic center where aggressive therapy like HCT is more likely to be considered.
References
Catenacci DV, Schiller GJ (2005) Myelodysplasic syndromes: a comprehensive review. Blood Rev 19(6):301–319. doi:10.1016/j.blre.2005.01.004
Garcia-Manero G (2014) Myelodysplastic syndromes: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol 89(1):97–108. doi:10.1002/ajh.23642
Adès L, Itzykson R, Fenaux P (2014) Myelodysplastic syndromes. Lancet. doi:10.1016/S0140-6736(13)61901-7
Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA et al (2008) Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 112(1):45–52. doi:10.1182/blood-2008-01-134858
McQuilten ZK, Wood EM, Polizzotto MN, Campbell LJ, Wall M, Curtis DJ et al (2014) Underestimation of myelodysplastic syndrome incidence by cancer registries: results from a population-based data linkage study. Cancer 120(11):1686–1694. doi:10.1002/cncr.28641
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114(5):937–951. doi:10.1182/blood-2009-03-209262
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G et al (1997) International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89(6):2079–2088
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F et al (2012) Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120(12):2454–2465. doi:10.1182/blood-2012-03-420489
Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R et al (2007) Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 25(23):3503–3510. doi:10.1200/JCO.2006.08.5696
Malcovati L, Della Porta MG, Strupp C, Ambaglio I, Kuendgen A, Nachtkamp K et al (2011) Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based Prognostic Scoring System (WPSS). Haematologica 96(10):1433–1440. doi:10.3324/haematol.2011.044602
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A et al (2009) Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 10(3):223–232. doi:10.1016/S1470-2045(09)70003-8
Ishikawa T (2014) Novel therapeutic strategies using hypomethylating agents in the treatment of myelodysplastic syndrome. Int J Clin Oncol 19(1):10–15. doi:10.1007/s10147-013-0651-5
Joeckel TE, Lübbert M (2012) Clinical results with the DNA hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in patients with myelodysplastic syndromes: an update. Semin Hematol 49(4):330–341. doi:10.1053/j.seminhematol.2012.08.001
McCormack SE, Warlick ED (2010) Epigenetic approaches in the treatment of myelodysplastic syndromes: clinical utility of azacitidine. Onco Targets Ther 3:157–165
Warlick ED, Smith BD (2007) Myelodysplastic syndromes: review of pathophysiology and current novel treatment approaches. Curr Cancer Drug Targets 7(6):541–558
Sorror M, Maris M, Storb R, Baron F, Sandmaier B, Maloney D et al (2005) Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106(8):2912–2919. doi:10.1182/blood-2005-05-2004
Pease DF, Ross JA, Poynter JN, Nguyen PL, Hirsch B, Cioc A et al (2015) Differences in community and academic practice patterns for newly diagnosed myelodysplastic syndromes (MDS) patients. Cancer Epidemiol 39(2):222–228. doi:10.1016/j.canep.2015.01.006
Oran B, Popat U, Andersson B, Champlin R (2013) Allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes. Clin Lymphoma Myeloma Leuk 13(Suppl 2):S282–S288. doi:10.1016/j.clml.2013.07.012
Warlick ED, Cioc A, Defor T, Dolan M, Weisdorf D (2009) Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant 15(1):30–38. doi:10.1016/j.bbmt.2008.10.012
Myelodysplastic syndromes (MDS). Retrieved from https://bethematchclinical.org/Transplant-Indications-and-Outcomes/Disease-Specific-Indications-and-Outcomes/MDS/. In
Spellman S, Setterholm M, Maiers M, Noreen H, Oudshoorn M, Fernandez-Viña M et al (2008) Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biol Blood Marrow Transplant 14(9 Suppl):37–44. doi:10.1016/j.bbmt.2008.05.001
Della Porta MG, Alessandrino EP, Bacigalupo A, van Lint MT, Malcovati L, Pascutto C et al (2014) Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood 123(15):2333–2342. doi:10.1182/blood-2013-12-542720
Komrokji RS, Padron E, Lancet JE, List AF (2013) Prognostic factors and risk models in myelodysplastic syndromes. Clin Lymphoma Myeloma Leuk 13(Suppl 2):S295–S299. doi:10.1016/j.clml.2013.05.022
Della Porta MG, Malcovati L, Strupp C, Ambaglio I, Kuendgen A, Zipperer E et al (2011) Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica 96(3):441–449. doi:10.3324/haematol.2010.033506
Naqvi K, Garcia-Manero G, Sardesai S, Oh J, Vigil CE, Pierce S et al (2011) Association of comorbidities with overall survival in myelodysplastic syndrome: development of a prognostic model. J Clin Oncol 29(16):2240–2246. doi:10.1200/JCO.2010.31.3353
Acknowledgments
The authors would like to acknowledge the support given by NIH grants R01 CA142714 and K05 CA157439 and the Minnesota Cancer Surveillance System.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, A.R., Warlick, E.D., Roesler, M.A. et al. Factors associated with hematopoietic cell transplantation (HCT) among patients in a population-based study of myelodysplastic syndrome (MDS) in Minnesota. Ann Hematol 94, 1667–1675 (2015). https://doi.org/10.1007/s00277-015-2422-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2422-z