Abstract
Acute myeloid leukemia (AML) survival rates in younger patients have improved considerably since the 1970s. In order to evaluate the impact of AML and its treatment on fertility and family situation in adult long-term survivors, we used the Swedish population-based registries to identify 161 adult patients diagnosed with AML within the Leukemia Group of Middle Sweden (LGMS) 1973–2003, who survived for more than 5 years and were alive in 2010. Ninety-eight patients (61 %) completed a questionnaire including items on reproductive concerns, family situation, and infertility-related distress. After excluding women >45 years and/or postmenopausal women and men >55 years, 22 women and 38 men were included in the final analysis. Nine of the women (41 %) tried to conceive after treatment, but only three succeeded. Five (83 %) of the unwillingly childless women reported “a moderate” or “a lot” of distress caused by this. Among men in the same age group, all six who wanted children after treatment succeeded. None of the men 46–55 years old cryopreserved their sperm or tried to father a child. Among patients who wanted children after AML treatment, 46 % of the women and 40 % of the younger men reported that they were not, or not fully, informed about fertility-related issues. In contrast, among men 46–55 years, none reported they would have wanted more information. Infertility among young female AML survivors thus remains an important clinical issue, and there is a need for improved clinical counseling and education in this area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The survival rates in acute myeloid leukemia (AML) are increasing, especially among young patients, who are at risk for potential long-term disease—and treatment-related side effects such as impaired reproductive ability [1, 2]. About 7 % of the adult patients are diagnosed in their childbearing age (18–44 years), corresponding to the fertile age in females [1]. Approximately 55 % of AML patients diagnosed in this age category are long-term survivors (surviving for more than 5 years after their diagnosis) [3].
The median age for women to give birth to their first baby steadily increases in developed countries [4], creating a larger proportion of young AML patients who have not yet had the children they want before they are diagnosed with AML and start chemotherapy. Little is known regarding fertility-related concerns and consequences in long-term adult AML survivors, as published reports mostly focus on the crude fertility rates [5–7], while perceptions of fertility issues have been investigated mainly in other cancer forms such as breast cancer, gynecological cancers, and lymphoma [8].
It has been established that chemotherapy, alkylating agents in particular, and radiotherapy included in pre-transplant conditioning before hematopoietic stem cell transplantation (HSCT) are potent threats for both female and male gonadal function [9–14]. Several studies show an increasing risk of infertility with intensified treatment, the highest infertility rates seen in allografted patients, followed by autografted patients and patients receiving chemotherapy only [15, 16]. In order to preserve reproductive possibilities, cryopreservation of sperm has been used for male patients for more than 30 years [5]. It is far more complicated to preserve female reproductive possibilities. The acute nature of the disease does not allow for a postponed start of chemotherapy in order to perform ovarian stimulation and oocyte collection for immediate in vitro fertilization (IVF) and embryo cryopreservation or, if the patient does not have a partner, oocyte cryopreservation. Ovarian tissue cryopreservation can, however, be performed without an unacceptable delay in starting chemotherapy, and more than 25 live births have so far been reported as the result of this technique mainly in patients treated for breast cancer and lymphoma [17–20]. Due to concern about residual disease in ovarian cortex biopsies from patients with leukemia, auto-reimplantation has so far only been reported in one leukemia patient (with CML), where the ovarian tissue graft did not show any evidence of leukemic cells when analyzed with highly sensitive RT-PCR [19]. It is also worth noting that some centers have performed oocyte collection for embryo banking between induction and consolidation chemotherapy in selected AML cases [21].
A recent Scandinavian study of childhood AML survivors has shown fertility outcomes comparable to their siblings, who were used as controls [22]. However, it is known that the risk of treatment-related infertility increases with age at time of treatment [12], and the literature on posttreatment fecundity in adult leukemia is scarce. A Norwegian population-based matched-control study on young cancer survivors (16–45 years at diagnosis) has shown a hazard ratio (HR), as compared to the control group, of pregnancy after acute leukemia of 0.55 (95 % confidence interval (CI) 0.38–0.80) for men and 0.37 (95 % CI 0.23–0.62) for women. Breast cancer, cervical cancer, and acute leukemia had the lowest HR of pregnancy of all cancer forms [5]. A recently published British study on fertility outcomes after treatment for hematological malignancies reported an OR of 2.23 of childlessness after acute leukemia compared to after Hodgkin’s lymphoma [23]. While posttherapy fecundity in men has risen considerably since the early 1980s, corresponding to the advent of cryopreservation of sperm, the posttherapy fertility in women remains unaltered [5].
The aim of this study was to assess the impact of AML and its treatment on fertility in adult AML long-term survivors and to what extent patients were affected by their infertility. Swedish population-based registries were used to identify those patients in their fertile years at the time of AML diagnosis who survived 5 years or more.
Methods
All adult patients diagnosed with AML during 1973–2003 within the area of the Leukemia Group of Middle Sweden (LGMS), including regions of Stockholm, Uppsala, and Örebro, were identified through the Swedish Cancer Registry. Patients who died within 5 years of AML diagnosis were identified through the Cause of Death Registry and excluded.
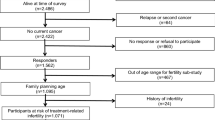
Out of 311 patients identified as long-term survivors, 176 were still alive at study start in May 2010. Seven patients were excluded due to reasons described in Fig. 1. A letter was sent to 169 patients explaining the objectives of the study and asking them to participate. One hundred and sixty-one were still alive and capable of participating by the time the questionnaires were received (Fig. 1). Patients who did not return the enclosed response form were reminded by a phone call or a letter. Once the patients had accepted to participate, they were sent a questionnaire by mail. Ninety-eight patients consented participation (response rate 61 %).
The current report focuses on fertility-related issues, and therefore, all female patients >45 years of age (n = 29) and women who had already reached menopause at the time of diagnosis (n = 3) were excluded, leaving 22 women in the study (response rate in this group 53 %). For men, we chose the upper age limit used by the Swedish National Board of Health and Welfare for male assisted fertilization, 55 years, leaving 38 men in the study (response rate in this group 62 %). We present the results separately for men ≤45 years (younger age group) and men 46–55 years (older age group), enabling a better comparison with the female patients.
Measurements and procedures
Data was collected by a self-reported questionnaire, validated for studies of quality of life, sexual function, and fertility in patients with other types of cancer [24–27]. Minor modifications were made to adapt it to the AML setting. The questionnaire included questions regarding age and family situation at the time of diagnosis, treatment, and in-depth questions about fertility including IVF and sperm cryopreservation. Several questions addressed the desire to become a parent and to what extent that had been altered after the disease and treatment. Possible distress caused by infertility was assessed on a verbal scale of intensity from “It does not distress me at all” and “It distresses me a little” to “It distresses me moderately” and “it distresses me a lot.” Psychological well-being and symptoms such as anxiety or depression were assessed on a Likert-type scale ranging from 1 = never to 7 = always, and means were calculated for comparison. There was also an open-ended question where patients were asked to describe the three worst consequences of the disease and treatment. All information about disease, treatment, and sociodemographics was obtained solely from the patients in order to safeguard their anonymity.
The study was approved by the Regional Ethics Review Board in Stockholm, Sweden, and written informed consent was obtained from all patients.
Results
Demographic and clinical data are presented in Table 1. The median age at diagnosis was 34 years for the female patients and 38 years in the younger male group. The median observation time after diagnosis was 15 years in both groups. Among patients ≤45 years old, there was a higher proportion of the men treated with allogeneic HSCT compared to women, 55 % (n = 12) and 27 % (n = 6), respectively. The same was true for autologous HSCT (men 23 %, n = 5 and women 9 %, n = 2). As a consequence, more men than women were treated with total body irradiation (Table 1). All of the allografted women and eight (67 %) of the allografted younger men reported that they had been or were still suffering from some degree of graft versus host disease.
Fertility outcome
At the time of AML diagnosis, 16 (73 %) of the women, 17 (71 %) of men ≤45 years, and 12 (86 %) of men 46–55 years old already had children. Four women (18 %) were pregnant at the time of diagnosis. One woman gave birth to a healthy girl shortly after diagnosis. The remaining three pregnancies were in earlier stages at the time of diagnosis and ended in miscarriages or abortions.
Thirteen women (59 %) reported that they did not want children after the disease and therefore did not try to conceive (Table 2). All women without children prior to AML diagnosis wished for children afterwards, whereas four out of seven men without children did not wish for children. Among women and men with three or more children, there was no wish for additional children after AML treatment.
After AML treatment, six women became pregnant resulting in three live births (Fig. 2). Two women had miscarriages, and one probably had an abortion. Women with successful pregnancies were all between 21 and 27 years old at the time of diagnosis and had received chemotherapy only. The pregnancies were all accomplished without any medical assistance. Three women tried to conceive but did not become pregnant. These three women were 22, 28, and 36 years old at the time of diagnosis and were all treated with allogeneic HSCT. One of them later adopted a child.
Among males ≤45 years at diagnosis, i.e., the younger male group, six men (25 %) had their sperm cryopreserved and two of them have fathered this way. Both of these men were treated with chemotherapy only. Another young man fathered a child from a pregnancy after medical assistance, though without previous banking of sperm. He stated he was infertile already before he was diagnosed with leukemia and therefore chose not to have his sperm cryopreserved. Three younger men fathered without medical assistance, one who was treated with chemotherapy only and two who also underwent autologous HSCT. None of the men, regardless of age, reported they have tried to father after their treatment without succeeding.
Two (11 %) of the women and two (9 %) of the younger men reported they had become reluctant/dubious about having children after their disease. An additional two (11 %) women and two (9 %) younger men reported that they did not any longer want to become parents after their disease. One (7 %) man 46–55 years old (already the father of three children) had an altered view on parenthood after the disease and did not want any more children after his disease.
Three (17 %) women and four (18 %) younger men reported that their desire of parenthood was unaffected by the disease and treatment.
Perceptions of information received about risk of infertility related to AML treatment
Among the patients that wanted children after AML treatment (here defined as not answering “not applicable/I haven’t wished for/tried to conceive” on the question “Have you had children after you were treated for AML?”), 6/13 women (46 %) and 6/15 in the younger male group (40 %) reported that they did not receive sufficient information about the risk of infertility associated with the treatment (Table 2). Only seven (35 %) of the women and nine (38 %) of the younger men (≤45 years old) reported that they had received sufficient information regarding fertility issues. None of the men 46–55 years old would have wanted more information.
Six of the younger men (25 %) reported they were informed about the risk of infertility and offered the possibility of cryopreservation of semen but declined. Ten younger men (42 %) reported they were never offered that possibility. None of the men 46–55 years old had their sperm cryopreserved, ten older men (71 %) reported they were not offered the possibility, and the rest declined.
Psychological well-being
Among patients who had attempted to have children and did not succeed, none of the women reported they were psychologically unaffected by this. Five out of six women reported that they were “moderately” or “a lot” distressed by this and one was “a little” distressed by her infertility. One man reported sperm banking was not offered at the time of diagnosis. He later insisted on having it done, after start of chemotherapy, but he believed it was probably too late. Because he was never in a relationship, he had never tried to use his cryopreserved semen; however, he was “a little” distressed by his infertility. One man, already the father of a child at the time of diagnosis, tried for 7 years after the treatment before he finally succeeded in fathering an additional child without medical assistance. He reported he was “moderately” distressed by his infertility. None of the men were “a lot” distressed by their infertility (Table 2).
The psychological well-being and the acceptance of the life situation, including family-related questions, were overall good. Women who tried to have children after treatment without succeeding (n = 6) showed no clear signs of psychological impairment. They scored well-being and presence of depression or anxiety slightly more favorable than the other women in the study (well-being mean 5.6 compared to 5.4 where 7 = excellent and 1 = very poor, and depression as well as anxiety 2.0 compared to 2.75 where 7 = all the time and 1 = never). However, a larger proportion of the women who tried to have children after treatment without succeeding stated they felt less attractive after the treatment (60 % versus 32 % of all women). Also, half of the women who had tried to have children after treatment without succeeding reported in the open-ended question asking for the three worst consequences of their disease that infertility was the single worst complication of their entire AML treatment. In addition, one woman who was pregnant at the time of diagnosis and gave birth to a healthy girl shortly thereafter reported that not being able to be a good mother was the worst part of the leukemia treatment period. None of the men reported that infertility was among the worst consequences of their disease and treatment.
Discussion
In this population-based study, we investigated fertility and psychological effects of impaired fertility among 60 long-term AML survivors in fertile age, belonging to a cohort constituting approximately 40 % of the AML long-term survivors in Sweden. We found that fertility is an important aspect for many AML survivors, especially among female patients where a number of them considered the therapy-related infertility to be the worst of all consequences of the AML disease and its treatment. Similar results have been published earlier for broader groups of cancer patients or hematological malignancies but not focusing on AML.
Despite a median age of 34 years at the time of AML diagnosis of the women in this study, 41 % had tried to conceive after AML treatment, but only a minority succeeded in giving birth to a child. None of the women treated with HSCT succeeded in conceiving, which is consistent with earlier data showing extremely low pregnancy rates after HSCT [28, 29]. When asked about what degree of distress their treatment-related infertility had caused them, a majority of the women who wanted children reported that it caused them “moderate” or “a lot” of distress; half of them even considered this as the worst of all disease consequences. However, there was no evidence of increased levels of anxiety or depression among women who were childless at diagnosis and who tried to conceive without succeeding, despite the fact that many of them reported that they were distressed by not having children.
The use of sperm cryopreservation among young men was consistent with earlier studies [30, 23]. Although our material is too small to make valid comparisons, a slightly higher proportion later used their cryopreserved sperm and fathered this way [31, 23]. In fact, all men in this study who tried to father after AML treatment succeeded. Thus, in contrast to the women, the younger men did not report pronounced distress related to infertility. Men between 46–55 years of age at the time of their AML diagnosis were not concerned with infertility. None of them had their sperm cryopreserved, and none of them tried to father after their treatment.
Factors other than infertility caused by chemotherapy and HSCT may contribute to the low fecundity in long-term AML survivors. Half of the women and younger men who wanted children before they were diagnosed with AML reported doubts about having children or did not want children at all after AML treatment. The reasons for this change in attitude were not covered by the questionnaire questions, but one could speculate that the considerable risk of relapse and worries about having children that would later loose a parent might contribute. The risk of relapse is regarded as very low after 5 years in complete remission, but by then, the chance of having a child may be smaller, especially for women, who have a high risk of premature ovarian failure. Fear of possible hereditary traits of leukemia [32] might be another reason survivors doubt having children after their treatment.
In contrast to our results, in a study of long-term female cancer survivors (not including AML patients), 68 % of women reported that the experience of cancer did not influence the desire for a child, 15 % reported an increased, and 17 % reported a decreased desire to have children [8]. Interestingly, in our study, all women without children prior to AML diagnosis wished for children afterwards, while only three out of seven men without children had the same wish. An additional finding is that 18 % of the women in this study were pregnant at the time of diagnosis. Although based on a small material, it further underlines the many aspects of fertility and parenthood that we face in AML treatment in fertile women.
In our study, only 35 % of the women and 38 % of the younger men reported that they had received sufficient information regarding fertility issues. There is evidence that receiving specialized counseling about reproductive loss and pursuing fertility preservation is associated with less regret and greater quality of life for survivors [33] even if no procedure to preserve fertility is currently possible due to the nature of the disease. Also, reimplantation of ovarian biopsies might be a possibility in the near future for younger women, as this technique evolves. Lack of information has been reported in other studies of cancer survivors [33, 34]. However, a recent Swedish study showed that leukemia patients were more likely to receive fertility counseling than, for example, breast cancer patients, probably as a result of their younger age [35].
A strength of our study is the population-based design which enables identification of all AML long-term survivors of fertile age within the selected regions. Thus, all patients fulfilling the inclusion criteria were asked to participate to reduce potential problems of selection. In addition, we used an anonymous questionnaire that was answered at home. This method probably results in fewer investigator-derived errors than, for example, a personal interview or an identifiable questionnaire [36, 37]. Indeed, the response rates in this study were 53 % for women and 62 % for men despite the extensive questionnaire also including questions regarding sexual problems (data to be published).
The main drawback of our study is the limited number of included patients in fertile age due to the high median age at AML diagnosis. A response bias related to negative or positive perceptions regarding the disease and its treatment cannot be excluded. In addition, some patients were diagnosed with AML a long time ago, and there may be an issue of recall bias especially concerning information given.
This is, to our knowledge, the first study describing the degree of distress infertility may cause AML survivors and to what extent AML and its treatment may alter the desire to become a parent. Also, the different perceptions of fertility in male AML patients of different age groups had not been described before. Since different cancer forms differ regarding disease presentation, it is of value to evaluate the leukemia-related aspects separately, as results from solid tumors are not always transferrable.
In our study, the fertility-related information given to AML patients in reproductive age was often perceived as insufficient. Young female AML survivors who did not succeed in having the children they wanted experienced considerable distress related to their infertility. Our study underlines the importance of fertility-related outcomes in AML patients and, as new methods of fertility preservation for leukemia patients are being developed especially for women, the great need for adequate information and counseling regarding fertility impairment and fertility preservation.
References
Derolf AR, Kristinsson SY, Andersson TM, Landgren O, Dickman PW, Bjorkholm M (2009) Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood 113(16):3666–3672. doi:10.1182/blood-2008-09-179341
Burnett AK (2005) The treatment of AML: current status and novel approaches. Hematology 10(Suppl 1):50–53. doi:10.1080/10245330512331389773
Adult acute leukemia, report number 6 (Blood Cancer Registry 2010). Swedish Hematology Association
Statistics Sweden. Demographic reports 2011
Stensheim H, Cvancarova M, Moller B, Fossa SD (2011) Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer J Int du Cancer 129(5):1225–1236. doi:10.1002/ijc.26045
Hartman M, Liu J, Czene K, Miao H, Chia KS, Salim A, Verkooijen HM (2013) Birth rates among female cancer survivors: a population-based cohort study in Sweden. Cancer 119(10):1892–1899. doi:10.1002/cncr.27929
Kawamura S, Suzuki Y, Tamai Y, Itoh J, Fukushima K, Takami H, Yoshida Y, Sawada Y, Sakata Y (1995) Pregnancy outcome among long-term survivors with acute leukemia. Int J Hematol 62(3):157–161
Canada AL, Schover LR (2012) The psychosocial impact of interrupted childbearing in long-term female cancer survivors. Psycho-Oncology 21(2):134–143. doi:10.1002/pon.1875
Leung W, Hudson MM, Strickland DK, Phipps S, Srivastava DK, Ribeiro RC, Rubnitz JE, Sandlund JT, Kun LE, Bowman LC, Razzouk BI, Mathew P, Shearer P, Evans WE, Pui CH (2000) Late effects of treatment in survivors of childhood acute myeloid leukemia. J Clin Oncol Off J Am Soc Clin Oncol 18(18):3273–3279
Bahadur G, Ozturk O, Muneer A, Wafa R, Ashraf A, Jaman N, Patel S, Oyede AW, Ralph DJ (2005) Semen quality before and after gonadotoxic treatment. Hum Reprod 20(3):774–781. doi:10.1093/humrep/deh671
Hallak J, Kolettis PN, Sekhon VS, Thomas AJ Jr, Agarwal A (1999) Cryopreservation of sperm from patients with leukemia: is it worth the effort? Cancer 85(9):1973–1978
Meirow D (2000) Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol 169(1–2):123–131
Gracia CR (2010) Reproductive health after cancer. Cancer Treat Res 156:3–9. doi:10.1007/978-1-4419-6518-9_1
Wallace WH (2011) Oncofertility and preservation of reproductive capacity in children and young adults. Cancer 117(10 Suppl):2301–2310. doi:10.1002/cncr.26045
Watson M, Wheatley K, Harrison GA, Zittoun R, Gray RG, Goldstone AH, Burnett AK (1999) Severe adverse impact on sexual functioning and fertility of bone marrow transplantation, either allogeneic or autologous, compared with consolidation chemotherapy alone: analysis of the MRC AML 10 trial. Cancer 86(7):1231–1239
Zittoun R, Suciu S, Watson M, Solbu G, Muus P, Mandelli F, Stryckmans P, Peetermans M, Thaler J, Resegotti L, Dardenne M, Willemze R (1997) Quality of life in patients with acute myelogenous leukemia in prolonged first complete remission after bone marrow transplantation (allogeneic or autologous) or chemotherapy: a cross-sectional study of the EORTC-GIMEMA AML 8A trial. Bone Marrow Transplant 20(4):307–315. doi:10.1038/sj.bmt.1700888
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A (2004) Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 364(9443):1405–1410. doi:10.1016/S0140-6736(04)17222-X
Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J (2005) Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 353(3):318–321. doi:10.1056/NEJMc055237
Meirow D, Raanani H, Brengauz M, Dor J (2012) Results of one center indicate that transplantation of thawed ovarian tissue is effective. Repeated IVF reveals good egg quality and high pregnancy rate. Meeting of the European Society of Human Reproduction and Embryology (ESHRE), Istanbul, Turkey
Chung K, Donnez J, Ginsburg E, Meirow D (2013) Emergency IVF versus ovarian tissue cryopreservation: decision making in fertility preservation for female cancer patients. Fertil Steril 99(6):1534–1542. doi:10.1016/j.fertnstert.2012.11.057
Rossi BV, Ashby RK, Srouji SS (2011) Embryo banking between induction and consolidation chemotherapy in women with leukemia. Fertil Steril 96(6):1412–1414. doi:10.1016/j.fertnstert.2011.09.038
Molgaard-Hansen L, Skou AS, Juul A, Glosli H, Jahnukainen K, Jarfelt M, Jonmundsson GK, Malmros J, Nysom K, Hasle H, Nordic Society of Pediatric H, Oncology (2013) Pubertal development and fertility in survivors of childhood acute myeloid leukemia treated with chemotherapy only: a NOPHO-AML study. Pediatr Blood Cancer 60(12):1988–1995. doi:10.1002/pbc.24715
Greaves P, Sarker SJ, Chowdhury K, Johnson R, Matthews J, Matthews R, Smith M, Korszun A, Gribben JG, Lister TA (2013) Fertility and sexual function in long-term survivors of haematological malignancy: using patient-reported outcome measures to assess a neglected area of need in the late effects clinic. Br J Haematol. doi:10.1111/bjh.12651
Bergmark K, Avall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G (1999) Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med 340(18):1383–1389. doi:10.1056/NEJM199905063401802
Helgason A, Adolfsson J, Dickman P, Fredrikson M, Steineck G (1998) Distress due to unwanted side-effects of prostate cancer treatment is related to impaired well-being (quality of life). Prostate Cancer Prostatic Dis 1(3):128–133. doi:10.1038/sj.pcan.4500226
Helgason AR, Adolfsson J, Dickman P, Fredrikson M, Arver S, Steineck G (1996) Waning sexual function—the most important disease-specific distress for patients with prostate cancer. Br J Cancer 73(11):1417–1421
Onelov E, Steineck G, Nyberg U, Hauksdottir A, Kreicbergs U, Henningsohn L, Bergmark K, Valdimarsdottir U (2007) Measuring anxiety and depression in the oncology setting using visual-digital scales. Acta Oncol 46(6):810–816. doi:10.1080/02841860601156124
Salooja N, Szydlo RM, Socie G, Rio B, Chatterjee R, Ljungman P, Van Lint MT, Powles R, Jackson G, Hinterberger-Fischer M, Kolb HJ, Apperley JF, Late Effects Working Party of the European Group for B, Marrow T (2001) Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet 358(9278):271–276
Carter A, Robison LL, Francisco L, Smith D, Grant M, Baker KS, Gurney JG, McGlave PB, Weisdorf DJ, Forman SJ, Bhatia S (2006) Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant 37(11):1023–1029. doi:10.1038/sj.bmt.1705364
Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S (2002) Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol Off J Am Soc Clin Oncol 20(7):1880–1889
Agarwal A, Ranganathan P, Kattal N, Pasqualotto F, Hallak J, Khayal S, Mascha E (2004) Fertility after cancer: a prospective review of assisted reproductive outcome with banked semen specimens. Fertil Steril 81(2):342–348. doi:10.1016/j.fertnstert.2003.07.021
Goldin LR, Kristinsson SY, Liang XS, Derolf AR, Landgren O, Bjorkholm M (2012) Familial aggregation of acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol Off J Am Soc Clin Oncol 30(2):179–183. doi:10.1200/JCO.2011.37.1203
Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, Melisko ME, Cedars MI, Rosen MP (2012) Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer 118(6):1710–1717. doi:10.1002/cncr.26459
Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, Rosenberg R, Przypyszny M, Rein A, Winer EP (2004) Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 22(20):4174–4183. doi:10.1200/JCO.2004.01.159
Armuand GM, Rodriguez-Wallberg KA, Wettergren L, Ahlgren J, Enblad G, Hoglund M, Lampic C (2012) Sex differences in fertility-related information received by young adult cancer survivors. J Clin Oncol Off J Am Soc Clin Oncol 30(17):2147–2153. doi:10.1200/JCO.2011.40.6470
Steineck G, Ahlbom A (1992) A definition of bias founded on the concept of the study base. Epidemiology 3(6):477–482
Steineck G, Kass PH, Ahlbom A (1998) A comprehensive clinical epidemiological theory based on the concept of the source person-time and four distinct study stages. Acta Oncol 37(1):15–23
Acknowledgements
We gratefully acknowledge all participating patients and all LGMS senior participants that contributed patients to this registry cohort under study, as well as Petra Janeld who administered the questionnaires and Ninni Petersen for administrative support.
The study was supported by grants from the Adolf H. Lundin Charitable Foundation and by the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet.
Conflict of interest
The authors have stated that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
EB and ÅRD contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Brånvall, E., Derolf, Å.R., Johansson, E. et al. Self-reported fertility in long-term survivors of acute myeloid leukemia. Ann Hematol 93, 1491–1498 (2014). https://doi.org/10.1007/s00277-014-2088-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-014-2088-y