Abstract
Human leukocyte antigen haploidentical hematopoietic stem-cell transplantation (haplo-HSCT) is associated with an increased risk of graft failure and severe graft-versus-host disease (GVHD). Mesenchymal stromal cells (MSCs) have been shown to support in vivo normal hematopoiesis and to display potent immunesuppressive effects. We cotransplanted the culture-expanded third-party donor-derived umbilical cord MSCs (UC-MSCs) in 50 people with refractory/relapsed hematologic malignancy undergoing haplo-HSCT with myeloablative conditioning. We observed that all patients given MSCs showed sustained hematopoietic engraftment without any adverse UC-MSC infusion-related reaction. The median times to neutrophil >0.50 × 109/L and platelet >20 × 109/L engraftment were 12.0 and 15.0 days, respectively. We did not observe an increase in severe acute GVHD (aGVHD) and extensive chronic GVHD (cGVHD), too. Grade II–IV aGVHD was observed in 12 of 50 (24.0 %) patients. cGVHD was observed in 17 of 45 (37.7 %) patients and was extensive in 3 patients. Additionally, only five patients (10.0 %) experienced relapse at a median time to progression of 192 days. The probability that patients would attain progression-free survival at 2 years was 66.0 %. The results indicate that this new strategy is effective in improving donor engraftment and reducing severe GVHD, which will provide a feasible option for the therapy of high-risk hematologic malignancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an effective therapeutic modality for a variety of malignant and nonmalignant disorders. However, fewer than 30 % of patients who require allogeneic HSCT have a human leukocyte antigen (HLA)-compatible sibling. In China, searching for HLA-matched donors is usually unsuccessful because no siblings are available for almost all young people. Fortunately, HLA haplo-identical hematopoietic stem cell transplantation (haplo-HSCT) provides a cure for patients with refractory/relapsed hematologic malignancy who are suitable for HSCT but lack an HLA-matched donor. To date, the results of haplo-HSCT have been disappointing and remain complicated by treatment-related mortality because of infection, bleeding, regimen-related toxicity, engraftment failure, and acute and chronic graft-versus-host disease (GVHD) [1–3]. Recently, several approaches, such as T cell or CD3/CD19 cell depletion, the use of a “megadose” of stem cells, and nonmyeloablative (NMA)-conditioning regimens, have been employed and have shown a significant decrease in GVHD [2, 3]. However, for patients with refractory/relapsed malignant disorders, NMA conditioning regimens may increase the incidence of tumor relapse and graft rejection and decrease the success rate of allogeneic haplo-HSCT.
Recently, some experimental and clinical data demonstrated that mesenchymal stem cells (MSCs) can support hematopoiesis, enhance the engraftment of HSCs, and reduce the incidence of GVHD following HSCT [4, 5]. MSCs are a heterogeneous subset of stromal stem cells that can self-renew and are multipotent. Furthermore, these cells express low levels of class I, but not class II, histocompatibility antigens and are not immunogenic in in vitro assays or preclinical models. MSCs have also been shown to suppress primary and ongoing mixed lymphocyte reactions [6, 7]. MSCs can be isolated from many adult tissues, including bone marrow (BM), periosteum, adipose tissue, fetal liver, cord blood, and umbilical cord (UC) tissues [8–10]. Currently, BM represents the major source of MSCs for cell therapy. However, the aspiration of BM involves invasive procedures, and the frequency and differentiation potential of BM-MSCs decreases significantly with age [11]. Recent studies have shown that a large number of MSCs can be easily isolated from the UC and collected using an accessible procedure [12]. Our recent data demonstrated that the haplo-HSCT combined with third-party donor-derived UC-MSCs for nine patients with refractory severe aplastic was not only safe and feasible but also effective for the improvement of donor engraftment and the lessening of severe GVHD (unpublished data). However, it remains unclear whether cotransplantation of UC-MSCs in haplo-HSCT recipients with refractory/relapsed hematologic malignancy can improve engraftment and reduce severe GVHD.
Herein, we report the results of our clinical trial of haplo-HSCT with myeloablative (MA) conditioning that was designed primarily to examine the safety and feasibility of the cotransplantation of culture-expanded third-party donor-derived UC-MSCs and donor HSCs in hematologic malignancy patients. Our secondary goal was to investigate the rates and kinetics of hematopoietic engraftment and the incidence and severity of GVHD.
Patients and methods
Patients and study design
Fifty patients with hematologic malignancy aged 9–58 (median 26 years), including 24 males and 26 females, who needed transplantation but lacked an HLA-identical sibling donor were enrolled in this study in our center from January 2007 to June 2012. The diagnoses were based on the French–American–British criteria and were confirmed by immunophenotype and cytogenetic analyses. Twenty-three patients had acute myelogenous leukemia (AML), 17 had acute lymphoid leukemia (ALL), 7 had non-Hodgkin lymphoma (NHL), and the other 3 patients had chronic myelogenous leukemia (CML) in blast crisis. Of the 50 patient/donor pairs, 19 (38.0 %) were mismatched in 3 HLA loci, 17 (34.0 %) were mismatched in 2 HLA loci, and 14 (28.0 %) were mismatched in 1 HLA locus [13, 14]. Detailed information about the patients is listed in Tables 1 and 2. The clinical protocol and consent forms were approved by the institutional review board for human investigation. Patients or their legal guardians provided written informed consent for their inclusion in the study. Patients were required to have responsive or nonprogressive disease and adequate visceral organ function, including a left ventricular ejection fraction of at least 50 %, forced expiratory volume in 1 s and diffusion of carbon monoxide >50 % of predicted, serum direct bilirubin <2.0 mg/dL, and an actual or calculated creatinine clearance >60 mL/min. Patients were excluded for major central nervous system dysfunction or active infection.
UC-MSCs preparation
UC-MSCs were purchased from the National Engineering Research Center of Cell Products, State Key Laboratory of Experimental Hematology. The immunophenotype of UC-MSCs included positivity for CD13, CD29, CD90, CD44, CD105 (SH2), CD106, CD73 (SH3), CD166, and HLA-ABC but negativity for CD14, CD34, CD38, CD45, CD31, and HLA-DR. CD106 and HLA-ABC were expressed at significantly lower levels than the other markers [12].
High-dose chemotherapy
The conditioning regimen was based on our previous protocol for HLA-identical sibling HSCT. Conditioning procedures included one of two regimens: (1) regimen 1, for patients with AML or CML in BC, 35 mg m−2 day−1 fludarabine (Flu) (days −10 to −8), 2 g m−2 day−1 cytarabine (Ara-C) (days −10 to −8), 0.8 mg/kg/6 h busulfan (Bu) (days −7 to −5), 300 mg m−2 day−1 teniposide (VM-26) (day −4), 1.8 g m−2 day−1 cyclophosphamide (Cy) (days −3, −2), and 5 mg kg−1 day−1 rabbit anti-human T-lymphocyte immunoglobulin (ATG) (days −4 to −1); or (2) regimen 2, for patients with ALL or NHL, total body irradiation (TBI) with 1.8 Gy m−2 day−1 (days −9 to −7), 35 mg m−2 day−1 Flu (days −7 to −5), 2 g m−2 day−1 Ara-C (days −7 to −5), 300 mg m−2 day−1 VM-26 (day −4), 1.8 g m−2 day−1 Cy (days −3, −2), and 5 mg kg−1 day−1 ATG (days −4 to −1) [15–17].
Allogeneic HSC infusion
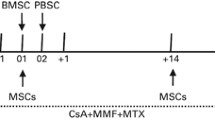
At the start of the study, BM and peripheral blood stem cells (PBSCs) (the percentages of BM and PBSCs were 50 %, respectively) were both elected for HSCT as the source of cellular rescue. Donor allogeneic HSCs were obtained using either a standard BM harvesting technique or a PBSC apheresis procedure with standard pheresis equipment (CS-3000 plus, Baxter, USA) after the subcutaneous administration of recombinant human granulocyte-colony stimulating factor (Kirin Brewery, Tokyo, Japan) 5 μg kg−1 day−1 for 5 days. For BM harvest and PBSC collection, a minimum of 6 × 108 mononuclear cells and a minimum of 2 × 106 CD34 cells were required, respectively. PBSCs were cryopreserved using a controlled-rate liquid nitrogen freezer. BM harvest cells were usually not cryopreserved. BM stem cells were infused intravenously through a central venous catheter on day 0 and thawed PBSCs were infused on day 1.
UC-MSC infusion
The planned UC-MSC dosing scheme was 5.0 × 105/kg in all patients. Before a planned UC-MSC infusion, the cells were shipped in liquid nitrogen containers to our transplantation center. Cryopreserved UC-MSC units were thawed at the bedside in a 37 °C sterile water bath and infused intravenously over 30 min as described previously [18]. BM infusions were performed 4 h after the completion of UC-MSC infusion [18]. Vital and clinical signs and symptoms were monitored at the time of infusion and every 15 min thereafter for 3 h, followed by every 2 h for 6 h and every 8 h for 3 days.
Prophylaxis and treatment of GVHD
GVHD prophylaxis consisted of intravenous cyclosporine 3 mg kg−1 day−1 in divided doses beginning the day before transplantation (day −3) and was continued thereafter. The oral mycophenolate mofetil (MMF) dose was 20 mg kg−1 day−1 from day −1 and was tapered off after 28 days if no acute GVHD (aGVHD) was observed. Rabbit ATG (Fresenius, Germany) was administered intravenously at a dose of 5 mg kg−1 day−1 only on days −4 to −1 before HSCT, and anti-CD25 antibody (Basiliximab, Novartis Pharma Stein AG, Switzerland) was administered intravenously at a dose of 0.5 mg kg−1 day−1 only on day 4 after HSCT. Patients were advanced to oral cyclosporine as tolerated. In the absence of GVHD, the oral cyclosporine dose was reduced weekly by approximately 5 % beginning on or near day 100, and therapy was usually discontinued by 6 months after transplantation. Acute and chronic GVHD were treated according to institutional practices.
Engraftment, toxicity grading, GVHD grading, and tumor assessment
All calculations were assessed from the day of HSC and UC-MSC infusions (designated as day 0). Neutrophil engraftment was defined as the first of three consecutive days of neutrophil counts >0.50 × 109/L, and platelet engraftment was defined as the first of seven consecutive days in which the platelet count exceeded 20 × 109/L without transfusion support. Toxic effects were graded using the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE version 4.0). Acute GVHD, which was defined as GVHD onset within 100 days of transplantation, was graded 0–IV (grades III to IV were considered moderate to severe) using a modification of the Glucksberg criteria [19]. Patients who survived more than 21 days after transplantation with evidence of engraftment were considered at risk for aGVHD. Chronic GVHD (cGVHD) was graded as limited or extensive according to previously published criteria in patients who survived more than 90 days with evidence of engraftment [20]. Patients underwent restaging with external imaging techniques and diagnostic marrow examinations as appropriate for each malignancy every 3 months after HSC infusion. Studies were not performed specifically to detect ectopic bone and cartilage formation. Relapse was defined as the recurrence of disease. Death in the absence of persistent relapse was categorized as nonrelapse mortality.
Supportive care
All patients had multilumen indwelling central venous catheters and were cared for in single rooms. Antibiotics were administered empirically for fever and neutropenia according to institutional guidelines, and all patients were supported with irradiated blood components in an attempt to maintain the hematocrit >25 % and the platelet count >10 × 109/L or to treat bleeding complications due to thrombocytopenia. Recombinant hematopoietic growth factors (Kirin Brewery, Tokyo, Japan) were administered routinely. Supportive care was given according to individual institutional practices and included cytomegalovirus infection prophylaxis with ganciclovir [21], Pneumocystis carinii pneumonia prophylaxis with trimethoprim-sulfamethoxazole, or inhaled pentamidine (in sulfa-allergic patients) at the start of the transplantation conditioning regimen. These treatments were withheld until the neutrophil count exceeded 0.50 × 109/L and were resumed until immunosuppressive therapy was discontinued. Antifungal agent prophylaxis included fluconazole, itraconazole, or liposomal amphotericin.
End points
Primary and secondary outcomes included the following factors: (1) determination of the short- and long-term safety of UC-MSC infusions, (2) donor-derived engraftment, (3) disease-/progression-free survival (i.e., survival without recurrent malignancy after transplantation), and (4) GVHD.
Statistical analyses
Statistical analysis was performed with SPSS 13.0 statistical software, version 18.0. Estimates of disease-/progression-free and overall survival were obtained using an analysis of life-table methods according to the Kaplan–Meier method.
Results
Engraftment and chimerisms
In Table 1, the median hematopoietic cell doses infused were 7.60 × 108 mononuclear cells (range, 5.95–9.74 × 108/kg) and 3.40 × 106 CD34+ cells (range, 1.92–14.42 × 106/kg) for transplants. Data for all patients who survived at least 30 days after transplantation and were evaluable for engraftment are summarized in Table 3. Overall, the median time to achieve neutrophil engraftment (absolute neutrophil count, ≥0.50 × 109/L) was 12.0 days (range, 9.0–20.0 days). Overall, the median time to achieve platelet engraftment ≥20 × 109/L was 15.0 days (range, 10.0–28.0 days). Delayed platelet recovery (>30 days) was not observed in any of the 50 patients. All 50 patients achieved full donor chimerism.
Graft-versus-host disease
Table 3 indicates the incidence and severity of GVHD. Overall, 21 of 50 (42.0 %) patients experienced aGVHD between 15 and 83 days after transplantation, including 9 (18.0 %) with grade I, 5(10.0 %) with grade II, 3 (6.0 %) with grade III, and 4 (8.0 %) IV aGVHD. The other 29 patients had no aGVHD. Seventeen of 45(37.7 %) evaluable patients who survived at least 90 days after transplantation experienced cGVHD, and which was limited in 14(31.1 %) patients and extensive in 3 (6.6 %) patients.
Relapse
Five of the 50 (10.0 %) patients experienced tumor relapse or progression at a median of 192 days (range, 65–385 days; Table 3). Two of these patients achieved a second complete remission after the withdrawal of immunotherapeutic agents: one of these patients survived, and the other patient later died of severe pulmonary fungal infection. The remaining one patient received regular chemotherapy with no responsiveness.
Survival
Upon follow-up for 1–58 months, 33 of the 50 (66.0 %) patients were alive well. As shown in Fig. 1, the probabilities of 1- and 2-year disease-/progression-free survival were 77.8 and 66.0 % in the 50 patients, respectively. Seventeen patients died, six patients died as a result of infection, seven patients died as a result of GVHD, and four patients died as a result of relapse. The probability of 2 year LFS in AML and CML patients was 75.4 %, which was significantly higher than that in the ALL and NHL patients (47.3 %, P = 0.024).
Clinical safety outcomes
As anticipated for the HSC transplant recipients that were given MA therapy, all 50 patients experienced at least one adverse event during the study period, and 39 of 50 (78.0 %) patients developed at least one grade IV adverse event (Table 4). Only nine patients (18.0 %) demonstrated adverse events that were considered treatment related (Table 5). However, no patients experienced infusional toxicity during the UC-MSC infusion.
Discussion
Some study reported that MSCs were infused into patients 1 or 24 h after BM or 4 h before BM infusion [18, 22]. In this study, there was no immediate or delay toxicity related to IV UC-MSCs. None of the 50 patients experienced allergic reactions or respiratory symptoms. Furthermore, in comparison with other reports [18, 22] in which large amounts (over 1 × 106/kg) of MSCs were usually infused intravenously, we administered 5 × 105 cells/kg of UC-MSCs to all of the patients in this study, and the clinical outcome was better, with milder GVHD and stable engraftment. Our results indicate that this dose of UC-MSCs retained an effective role.
Haplo-HSCT is still largely disappointing mainly because of high mortality from T cell-mediated alloreactions in lethal GVHD even T cells depletion or “megadose” of stem cells are used [2, 23]. In our center, the results of haplo-HSCT were very disappointed from January 2006 to December 2007, too. Fifteen patients received haplo-HSCT with the same GVHD prophylaxis except UC-MSCs. The incidences for grades II to IV and III/IV aGVHD was up to 66.7 and 40 %, respectively. At the same time, extensive cGVHD occurred in 4 (36.3 %) of 11 evaluable patients who survived at least 90 days after transplantation. The most disappointed results were that only seven (46.7 %) patients were alive in the following 24 months. In this present trial, all of the 50 patients received haplo-HSCT with the GVHD prophylaxis and only 14.0 % of them developed grade III-IV aGVHD, although the overall rate of aGVHD was 42.0 %, which was better than our previous results. Additionally, cGVHD occurred in 17 patients (limited, n = 14; extensive, n = 3). Many studies have shown that the incidences for grades II–IV and III/IV aGVHD range from 44 to 64 % and from 16 to 26 %, respectively. Extensive cGVHD rates range from 30 to 46 % [16, 24, 25]. Acute and chronic GVHD rates identified in the trial fall within these ranges and would be a little better than the abovementioned. The results indicate that the new GVHD prophylaxis with ATG, CD25, CSA, MMF, and UC-MSCs was effective for the lessening of lethal aGVHD and extensive cGVHD. Some studies have been well investigated that ATG may remain in vivo for a much longer time, which is beneficial for in vivo T cell depletion and consequently for prevention of aGVHD, whereas anti-CD25 antibody has similar capacity in treating and preventing GVHD [26]. UC-MSCs do not express the immune costimulatory molecules or HLA-DR and express low levels of HLA-ABC [12] and which possess inhibitory effects on a variety of immune cells (T cells, B cells, NK cells, and DC) in terms of their proliferation, differentiation, maturation, and secretion [27]. Le Blanc reported that refractory GVHD was significantly alleviated in a patient who received third-party donor-derived MSCs. Le Blanc et al. [5, 28] also demonstrated that the infusion of MSCs was an effective therapy for patients with steroid-resistant GVHD in a phase II clinical trial. Similar results were also observed in our recent studies of nine patients with severe aplastic anemia of haplo-HSCT, which showed that the cotransplantation of HSCs with third-party donor-derived UC-MSCs had much sustained donor engraftment and milder GVHD (unpublished data). All of these results suggest that the infusion of third-party donor-derived UC-MSCs may play a considerable role in the lessening of severe GVHD. In addition, seven patients died as a result of gut GVHD in the study. Therefore, we should use UC-MSC not only for prevention of GVHD but also for therapy of GVHD in the short future. A randomized double-blind clinical trial evaluating the use of third-party donor-derived UC-MSC infusion for the prevention and lessening or treatment of GVHD will be required to establish the efficacy of this treatment regimen.
High-dose immunosuppressive agents are usually employed to overcome the HLA barrier. However, the benefit of a decrease in the incidence of GVHD is often accompanied by the cost of higher rates of graft rejection, leukemia relapse, and infection, particularly for patients with refractory/relapsed hematologic malignancy [2, 3, 29]. In this study, all patients achieved sustained full donor engraftment and prompt hematopoietic recovery. The median times to a neutrophil recovery of 0.50 × 109/L and a platelet recovery 20 × 109/L for all patients were 12.0 days (range, 9.0–20.0 days) and 15 days (range, 10–28 days), respectively. Some studies have suggested that MSCs produce cytokines that support hematopoiesis, which could potentially enhance marrow recovery [12, 30, 31]. The results of this study suggest that the MA conditioning regimen was sufficient to achieve sustained donor engraftment in which the third-party donor-derived UC-MSCs may have played an important role.
In vitro, MSCs suppress T cell proliferation both by cell/cell interactions and via soluble factors [32–34]. Conversely, suppressed T cell function has the potential to abrogate graft-versus-leukemia activity in the allograft setting. Several groups have demonstrated that BM-MSCs facilitate tumor growth in in vitro and preclinical models [35]. In this study, we did not observe an increase in the number of patients who developed tumor progression. Fortunately, all 50 patients had a low relapse rate (10.0 %) and a high 2-year survival (66.0 %), among whom 23 AML and 3 CML patients had a higher 2-year survival rate (75.4 %) than that in the 17 ALL and 7 NHL patients (47.3 %), despite all of them belonged to refractory/relapsed hematologic malignancy. At the same time, interesting observation is that none of the patients who relapsed developed extensive cGVHD. This result was more favorable than those of other studies that used haploidentical transplantation by MA or NMA conditioning [36, 37].
In conclusion, our data demonstrate that the haplo-HSCT combined with third-party donor-derived UC-MSCs for patients with refractory/relapsed hematologic malignancy was not only safe and feasible but also effective for the improvement of donor engraftment and the lessening of severe GVHD. Therefore, this treatment regimen may provide a feasible option for the treatment of high-risk hematologic malignancy.
References
Tabbara IA, Zimmerman K, Morgan C, Nahleh Z (2002) Allogeneic hematopoietic stem cell transplantation: complications and results. Arch Intern Med 162(14):1558–1566
Rizzieri DA, Koh LP, Long GD, Gasparetto C, Sullivan KM, Horwitz M, Chute J, Smith C, Gong JZ, Lagoo A, Niedzwiecki D, Dowell JM, Waters-Pick B, Liu C, Marshall D, Vredenburgh JJ, Gockerman J, Decastro C, Moore J, Chao NJ (2007) Partially matched nonmyeloablative allogenetic transplantation: clinical outcome and immune reconstitution. J Clin Oncol 25(6):690–697
Bethge WA, Haegele M, Faul C, Lang P, Schumm M, Bornhauser M, Handgretinger R, Kanz L (2006) Haploidentical allogeneic hematopoietic cell transplantation in adult with reduced-intensity conditioning and CD3/CD19 depletion: fast engraftment and low toxicity. Exp Hematol 34(12):1746–1752
Angelopoulou M, Novelli E, Grove JE (2003) Cotransplantation of human mesenchymal stem cells enhances humanmyelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol 31(5):413–420
Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringdén O (2004) Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363(9419):1439–1441
Anker I‘t A, Noort WA, Scherjon SA, van der Kleijburg-Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE (2003) Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 88(8):845–852
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99(10):3838–3843
In’t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE (2003) Mesenchymal stem cells in human secondtrimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 88(8):845–852
Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH (2004) Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 103(5):1669–1675
Romanov YA, Svintsitskaya VA, Smirnov VN (2003) Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells 21(1):105–110
Rao MS, Matton MP (2001) Stem cells and aging: expanding the possibilities. Mech Ageing Dev 122(7):713–734
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Gong W, Han ZB, Xu ZS, Lu YX, Liu D, Chen ZZ, Han ZC (2006) Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 91(8):1017–1026
Mickelson E, Smith A, McKinney S, Anderson G, Hansen JA (1993) A comparative study of HLA-DRB1 typing by standard serology and hybridization of non-radioactive sequence-specific oligonucleotide probes to PCR-amplified DNA. Tissue Antigens 41(2):86–93
Petersdorf EW, Smith AG, Mickelson EM, Martin PJ, Hansen JA (1991) Ten HLA-DR4 alleles defined by sequence polymorphisms within the DRB1 first domain. Immunogenetics 33(4):267–275
Copelan EA, Biggs JC, Szer J, Thompson JM, Crilley P, Brodsky I, Klein JL, Kapoor N, Harman GS, Avalos BR (1993) Allogeneic bone marrow transplantation for acute myelogenous leukemia, acute lymphocytic leukemia, and multiple myeloma following preparation with busulfan and cyclophosphamide (BuCy2). Semin Oncol 20(4 suppl 4):33–38
Socié G, Clift RA, Blaise D, Devergie A, Ringden O, Martin PJ, Remberger M, Deeg HJ, Ruutu T, Michallet M, Sullivan KM, Chevret S (2001) Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. Blood 98(13):3569–3574
Blume KG, Kopecky KJ, Henslee-Downey JP, Forman SJ, Stiff PJ, LeMaistre CF, Appelbaum FR (1993) A prospective randomized comparison of total body irradiation-etoposide versus busulfan-cyclophosphamide as preparatory regimens for bone marrow transplantation in patients with leukemia who were not in first remission: a Southwest Oncology Group study. Blood 81(8):2187–2193
Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM (2000) Rapid hematopoietic recovery after co-infusion of autologous culture-expanded human mesenchymal stem cells (hMSCs) and PBPCs in breast cancer patients receiving high dose chemotherapy. J Clin Oncol 18(2):307–316
Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Doney K, Farewell V (1986) Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 314(12):729–735
Atkinson K, Horowitz MM, Gale RP, Lee MB, Rimm AA, Bortin MM (1989) Consensus among bone marrow transplanters for diagnosis, grading and treatment of chronic graft-versus-host disease. Bone Marrow Transplant 4(3):247–254
Goodrich JM, Bowden RA, Fisher L, Keller C, Schoch G, Meyers JD (1993) Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med 118(3):173–178
Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson SL, Laughlin MJ, Loberiza FR Jr, Moseley AB, Bacigalupo A (2005) Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 11(5):389–398
Handgretinger R, Chen X, Pfeiffer M, Mueller I, Feuchtinger T, Hale GA, Lang P (2007) Feasibility and outcome of reduced intensity conditioning in haploidentical transplantation. Ann N Y Acad Sci 1106:279–289
Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, Kashyap A, Flowers ME, Lilleby K, Chauncey TR, Storb R, Appelbaum FR (2001) Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med 344(3):175–181
Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, Shore TB, Walker IR, Browett P, Messner HA, Panzarella T, Lipton JH (2002) A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 100(5):1525–1531
Deeg HJ (2007) How I treat refractory acute GVHD. Blood 109(10):4119–4126
Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE (2006) Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in an onmyeloablative setting. Blood 108(6):2114–2120
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O (2008) Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371(9624):1579–1586
Spitzer TR (2005) Haploidentical stem cell transplantation: the always present but overlooked donor. Hematology Am Soc Hematol Educ Program 390–395
Koc ON, Lazarus HM (2001) Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant 27(3):235–239
Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, Gerson SL, Lazarus HM, Cairo M, Stevens CE, Rubinstein P, Kurtzberg J (2001) Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med 344(24):1815–1822
Rasmusson I, Ringden O, Sundberg B, Le Blanc K (2003) Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 76(8):1208–1213
Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F (2003) Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101(9):3722–3729
Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, Koc ON (2004) Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant 33(6):597–604
Dalton WS, Hazlehurst L, Shain K, Landowski T, Alsina M (2004) Targeting the bone marrow microenvironment in hematologic malignancies. Semin Hematol 41(2 suppl 4):1–5
Lee CK, deMagalhaes-Silverman M, Hohl RJ, Hayashi M, Buatti J, Wen BC, Schlueter A, Strauss RG, Gingrich RD (2003) Donor T-lymphocyte infusion for unrelated allogeneic bone marrow transplantation with CD3+ T-cell-depleted graft. Bone Marrow Transplant 31(2):121–128
Fung HC, Stein A, Slovak M, O’donnell MR, Snyder DS, Cohen S, Smith D, Krishnan A, Spielberger R, Bhatia R, Bhatia S, Falk P, Molina A, Nademanee A, Parker P, Rodriguez R, Rosenthal J, Sweetman R, Kogut N, Sahebi F, Popplewell L, Vora N, Somlo G, Margolin K, Chow W, Smith E, Forman SJ (2003) A long-term follow-up report on allogeneic stem cell transplantation for patients with primary refractory acute myelogenous leukemia: impact of cytogenetic characteristics on transplantation outcome. Biol Blood Marrow Transplant 9(12):766–771
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Wu and Z. Wang contributed equally to this study and should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Wu, Y., Wang, Z., Cao, Y. et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells with a myeloablative regimen for refractory/relapsed hematologic malignancy. Ann Hematol 92, 1675–1684 (2013). https://doi.org/10.1007/s00277-013-1831-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-013-1831-0