Abstract
We conducted a phase 2 study with bortezomib, doxorubicin, and dexamethasone (PAD) followed by thalidomide and dexamethasone (TD) in patients with relapsed multiple myeloma (MM). Forty patients were enrolled between November 2005 and October 2007, with follow-up continuing until January 2009. Efficacy could be assessed in 37 patients. The overall response rate to PAD followed by TD was 83.6%: complete response 51.4%, near-complete response 13.4%, very good partial remission 5.4%, and partial response 13.4%. The median follow-up was 27 months (range 13–39). The median progression-free survival (PFS) from the start of treatment was 18 months (95% CI, 9.7–26.2 months), with a 1-year PFS rate of 56.9% and 3-year PFS rate of 25.7%. Median overall survival was 35.1 months (95% CI, 18.5–51.7), with a 1-year survival rate of 75% and 3-year survival rate of 27.3%. One hundred seventy-eight PAD cycles (median 6, range 1–6) in 38 patients were assessable for safety. The most common hematologic toxicity was thrombocytopenia, with grade 3–4 in 35.8%. Sensory neuropathy occurred at grade 2 in 26.3% and grade 3 in 10.3%. Two hundred TD treatment cycles (median 4, range 0–12 cycles) were administered. Most adverse events were of mild degree and manageable. PAD followed by TD in patients with relapsed MM is very effective and tolerable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma (MM) is a malignancy of the plasma cells that accounts for approximately 1% of all cancers. Increasing insight into its biology and the availability of new therapeutic options have led to significant changes in the treatment of MM in recent years, resulting in improved survival. New treatment modalities include reduced intensity transplantation and a number of chemotherapeutic agents, of which thalidomide, immunomodulatory drugs such as thalidomide and lenalidomide, and proteasome inhibitors such as bortezomib are particularly promising [1–3]. Despite important advances, MM remains incurable with a median survival of approximately 30–36 months except for younger patients treated with autologous transplantation who have a median survival of approximately 5 years. Moreover, because MM is primarily a disease of elderly patients with a median age of 65 years at diagnosis, such aggressive treatment is not possible in more than half of the patients [4].
The treatment of patients with relapsed or refractory MM remains as a significant challenge. In this situation, the choice of the salvage therapy depends on many factors, such as the components of the initial therapy—including whether or not the patient has received high-dose therapy/autologous stem cell transplantation, the degree and duration of response to primary therapy, performance status and age at the time of relapse, type of the relapse, and previous toxicity. However, the management of relapsed and refractory MM has changed considerably. This change is primarily due to the introduction of novel agents into clinical practice. Thalidomide, the first of the immune modulators, is active in relapsed or refractory MM. When used as a single agent, thalidomide has response rates of 25% to 36% in patients with relapsed or refractory MM [5, 6]. Thalidomide used in combination with dexamethasone has response rates of 59% to 72% [7, 8].
Bortezomib is the most active single drug in treating relapsed or refractory MM. In the uncontrolled multiple myeloma managed with proteasome inhibition therapy (SUMMIT) trial, bortezomib produced a response rate of about 35% in patients with advanced or refractory MM [9]. The proteasome inhibition for extending remission (APEX) trial showed that bortezomib was superior to dexamethasone in response rate, progression-free survival (PFS), and overall survival (OS) [3]. An extended follow-up of this study has shown that the overall response rate (ORR) and complete response (CR) rates for bortezomib were 43% and 9%, respectively, and that higher response quality was associated with longer response duration [10].
A large phase 3 trial comparing bortezomib and pegylated liposomal doxorubicin vs. bortezomib alone showed that bortezomib in combination is thought to be superior to bortezomib alone with regard to CR and very good partial remission (VGPR), time to progression, and OS [11]. Bortezomib has shown activity in relapsed or refractory disease, even in disease that is refractory to thalidomide [3, 9]. By acting through molecular mechanisms different from those of conventional chemotherapy or steroids, bortezomib has the potential to act synergistically with either of them and with other novel agents, including thalidomide and immunomodulatory analogs [12, 13]. We evaluated the efficacy and safety of bortezomib, doxorubicin, and dexamethasone (PAD) followed by thalidomide and dexamethasone (TD) in patients with relapsed or refractory MM (protocol number NCT00319865, KMM55).
Patients and methods
Patients
Patients with confirmed MM and measurable disease (a monoclonal immunoglobulin spike on serum electrophoresis ≥1 g/L and/or a urine monoclonal immunoglobulin spike ≥400 mg/24 h) were eligible. The disease must have progressed after a response to one or more lines of therapy or was refractory to initial treatment. The participants had an Eastern Cooperative Oncology Group performance status of 0 to 2, platelets at or higher than 75,000/mm3, hemoglobin at or higher than 8.0 g/dL, absolute neutrophils at or higher than 1,000/mm3, and creatinine clearance at or higher than 30 mL/min. Patients were bortezomib naïve. Additional exclusion criteria included known refractoriness to thalidomide, prior doxorubicin exposure more than 400 mg/m2, clinically significant cardiac disease, a left ventricular ejection fraction less than institutional normal limits, or grade 2 or higher peripheral neuropathy. All patients provided written informed consent before entering the study. The study protocol was approved by all institutional review boards and was conducted in accordance with federal regulations and the declaration of Helsinki.
Study design
This open-label, multicenter phase 2 study was conducted at 15 sites in South Korea. The primary endpoint was the response rate to PAD in relapsed MM. The secondary endpoints were as follows: PFS, defined as the time from enrollment to progression or death from any cause; OS, defined as the interval from enrollment to death from any cause; and toxicity profiles of PAD/TD.
Treatment plan and dose modification
Treatment consisted of six 21-day cycles of bortezomib 1.3 mg/m2 intravenously on days 1, 4, 8, and 11; doxorubicin 4.5 mg/m2 intravenously on days 1–4; and dexamethasone 40 mg orally on days 1–4. Dose reduction was allowed after the first cycle. Response was assessed at the end of two cycles. After receiving six cycles of PAD, responders received 12 cycles of TD (100 mg thalidomide days 1–28 and 40 mg dexamethasone days 1–4, every 28 days) as a consolidation treatment. In patients with progression during PAD therapy, the regimen was changed to 12 cycles of 200 mg thalidomide days 1–28 and 40 mg dexamethasone days 1–4, every 28 days. Thalidomide (100 mg) per day was given to the patients who did not achieve complete response at the end of TD, as a maintenance therapy until the disease progressed. Transfusion support and granulocyte growth factors were allowed. Thromboprophylaxis was not mandated during this study. Study treatment continued until disease progression or unacceptable treatment-related toxicity. Adriamycin and bortezomib could be held during cycle 1 for neutropenia or thrombocytopenia greater than grade 3. Bortezomib dose reductions were stipulated for grade 3 thrombocytopenia, grade 2 peripheral neuropathy: level 1, 1.0 mg/m2; level 2, 0.7 mg/m2.
Assessments
Patients were evaluated within 28 days before entering the study. Monitored MM parameters included β2-microglobulin, serum albumin, lactate dehydrogenase, serum protein electrophoresis, 24-hour urine collection for total protein and urine protein electrophoresis, and myeloma typing of serum and urine.
Responses were assessed based on serum and urine monoclonal (M) protein levels after cycle 2 and every other cycle thereafter. Response to treatment, assessed at the time of maximal response, was defined according to the EBMT criteria [14]. Furthermore, we included two other categories: near-complete response (nCR), defined by the absence of the M protein on electrophoresis with positive immunofixation; and VGPR, greater than 90% reduction in M protein. CR required confirmed disappearance of the monoclonal protein in the serum and urine by immunofixation studies and less than 5% plasma cells on bone marrow examination. Partial response (PR) was defined by at least a 50% reduction in the level of the serum M protein and a reduction in 24-hour urinary M protein of at least 90% or to less than 200 mg, plus no increase in the number or size of lytic bone lesions or any other evidence of disease progression by other parameters. Stable disease (SD) was defined as no change in M protein, while progressive disease (PD) was defined as recurrence of disease after CR or a greater than 25% increase in M protein from its lowest point. Adverse events were assessed according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0 [15].
Statistical analysis
The power analysis was based on the hypothesis that PAD followed by TD is 20% more effective (60%) than bortezomib only (40%) in terms of response rate. The study required 42 patients by Flemming’s single-stage design to keep the probabilities of type I and II errors at or below 0.05 and 0.2, respectively.
All enrolled patients were included in the intention-to-treat analysis of efficacy. The chi-square test was used to analyze response rate. PFS and OS were estimated using the Kaplan–Meier method and the curves were compared by the log-rank test.
Results
Patient characteristics
Forty patients were enrolled between November 2005 and October 2007, with follow-up continuing until January 2009. Patient and disease characteristics are listed in Table 1. At study entry, the median time from diagnosis was 30.2 months (range 5.1–111.5 months) and the median number of prior therapy regimens was 2 (range 1–5). The median age was 62 (range 41–75) years, and 55% of patients had a diagnosis of Durie–Salmon stage IIIA and IIIB MM at the time of registration. Using the International Staging System, based on serum β2-microglobulin and albumin levels, there were 13 patients with stage I, 11 patients with stage II, and 14 patients with stage III disease. The M immunoglobulin consisted of IgG (24 patients), IgA (seven patients), IgD (two patients), and light chain (five patients). The median β2-microglobulin was 3.5 mg/L, and the median albumin level was 3.6 g/dL. Among the participants, 72.5% were classified as having relapsed disease, and 37.5% had received more than three chemotherapy regimens. Cytogenetic aberrations were detected in 11 (42%) of 26 patients.
Twenty-three patients (57.5%) had previously undergone autologous stem cell transplantation, 33 (82.5%) had previously received anthracycline, and 13 (32.5%) had previously received thalidomide-based therapy.
Efficacy and survival
Three of the 40 patients enrolled in the protocol were excluded from the study after one cycle of treatment: two for protocol violation, and one died of a myocardial infarction. Thirty-seven patients were assessable for response, and the rates of different levels of response are shown in Fig. 1. After two cycles of PAD, 26 patients showed a response, and 16.2% of these had a CR. The ORR to six cycles of PAD was 81% with a CR rate of 32.4%. Of 25 patients with TD, seven had further improvement in status to a CR. Overall response to PAD followed by TD was 83.6%: CR 51.4%, nCR 13.4%, VGPR 5.4%, PR 13.4%; the SD and PD rates were both 2.8%. Figure 1 shows the cumulative responses after each cycle of treatment.
Response rates (%). Column 1 response rates after two cycles of PAD. Column 2 response rates after six cycles of PAD. Column 3 response rates after PAD and TD. CR complete response, nCR near-complete response, VGPR very good partial response, PR partial response, SD stable disease, PD progressive disease
Among the 23 patients whose disease had relapsed after an autotransplant, 13 (56%) achieved a CR, three (13%) had an nCR, and three (13%) had a more than PR. Among 13 patients who had prior thalidomide therapy, six (46%) achieved a CR and five (38%) had an nCR or PR. Cytogenetic aberrations were detected in 11 (42%) of 26 patients. Two groups exhibited similar high-quality responses (i.e., 73.3% in cytogenetic normal group vs. 81.8% in the cytogenetic aberration group). These responses were not frontline responses.
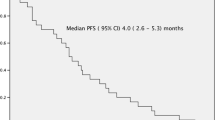
The median follow-up period was 27 months (range 13–39 months). A total of 25 patients relapsed and 17 died (one of myocardial infarction, one of septic pneumonia, and 15 of disease progression). The median PFS from study treatment was 18.0 months [95% confidence interval (CI), 9.7–26.2 months], with a 1-year PFS rate of 56.9% and 3-year PFS rate of 25.7% as shown in Fig. 2. The median OS was 35.1 months (95% CI, 18.5–51.7 months), with a 1-year survival rate of 75%, a 2-year survival rate of 61.5%, and a 3-year survival rate of 27.3% in Fig. 3. The median OS from first MM diagnosis was 112.9 months (95% CI, 24.6–201.1 months).
Adverse events
Overall, we administered 178 cycles of PAD (median 6, range 1–6). One myocardial infarction death occurred after the one cycle of PAD therapy. The median dose intensity was 1.38 mg/m2/week (range 0.54–1.84) for bortezomib and 5.25 mg/m2/week (range 1.83–6.63) for doxorubicin, which corresponds to 79.6% and 86.8% of the planned dose intensities, respectively. Twenty-five patients received TD. A total of 200 treatment cycles (median 4, range 0–12 cycles) was administered. One patient died of septic pneumonia.
The frequencies of hematologic and non-hematologic adverse events are shown in Table 2. The most common treatment-related hematologic adverse events during PAD treatment were thrombocytopenia (61.5%) and anemia (46.2%), with thrombocytopenia higher than grade 3 occurring in 35.8% of patients and anemia higher than grade 3 occurring in 10.3% of patients. Grade 3–4 neutropenia was observed in 7.7% of patients; however, no patients experienced febrile neutropenia. During TD treatment, the most common hematologic toxicity was neutropenia (20.0%); no patient experienced febrile neutropenia.
The frequencies of non-hematological adverse events are also shown in Table 2. Peripheral neuropathy was relatively common, occurring at grade 2 or higher in 36.6% of patients during PAD and 24% during TD. A total of 22.5% patients who received PAD treatment experienced at least one infectious episode, of which most were viral or bacterial infections of the upper respiratory tract, and 20% of patients developed grade 2 infections. Toxicity attributable to doxorubicin and bortezomib included diarrhea (worse than grade 2) in 25% of patients and stomatitis in 12.5% of patients.
During TD treatment, one patient died of septic pneumonia. Most side effects were mild or moderate, but toxicity in TD treatment was relatively infrequent.
Discussion
The goals of treatment for relapsed or refractory MM are to control disease and maintain quality of life. In selecting the optimal therapy, one must weigh the unique needs of individual patients against available evidence for the activity and safety of a given regimen. Given the host of treatment options available, various factors should be considered, including the influence of prior therapy, optimal sequencing of regimens, sequential versus combination use of agents, and the role of cytogenetic and other prognostic factors.
No studies have prospectively addressed the issue of optimal sequencing of agents, and the available information does not indicate which agents are preferable for treatment of a first relapse. Evidence from the APEX and MM-008/010 trials suggests that there is a benefit from using the more active drugs early in treatment and that bortezomib is more active when used earlier with relapses of MM. These conclusions were indicated by a longer OS and higher response rate among patients who had only one prior therapy compared with those who had more than one prior therapy [3, 16, 17]. In addition, no information is available regarding the effectiveness of alkylating agents or thalidomide following bortezomib or lenalidomide.
Combining agents with different mechanisms of action may potentially improve efficacy. As bortezomib and thalidomide target different molecular pathways, combining both with chemotherapy has resulted in remarkably high response rates. A previous study compared the results achieved in two groups of patients with relapsed or refractory MM receiving the same PTD regimen with or without liposomal doxorubicin. The ORR (81% vs. 50%), PFS (15 months vs. 8 months), and median OS (not reached vs. 13 months) were significantly higher with liposomal doxorubicin plus PTD [18]. To our knowledge, our study is the first to evaluate the sequential and combination efficacy of PAD followed by TD in relapsed MM. In our study, responses were achieved rapidly after the two cycles of PAD, with the best response achieved after four cycles in the majority of patients. The overall response rate to six cycles of PAD was 81% with a CR rate of 32.4%. TD showed further improvement of CR status in seven additional patients. Additional CR/nCR responses could be achieved potentially by the addition of TD. The following outcome measures were higher in our study of PAD followed by TD than in the study of liposomal doxorubicin plus PTD: CR plus nCR rate (64.8% vs. 52%), ORR (83.6% vs. 81%), median PFS (18 months vs. 15 months), and median OS (35.1 months vs. not reached) [18].
Briefly, the combination of active agents shows promise in increasing the depth (CR/VGPR) and durability of responses and potentially improving survival. Evidence suggests that the quality of response translates into survival benefit for patients with relapsed MM [10]. Improvement of CR or VGPR may be an important study endpoint because these are variables significantly associated with longer survival [19–22].
Response duration is another critical factor in evaluating newer therapies for relapsed or refractory MM. In the initial trials of single-agent bortezomib, the median time to progression was approximately 6 months. PAD was assessed for the first time in the treatment of relapsed/refractory MM, and the median event-free survival was 9 months [23]. In our study, the median PFS was 18 months (95% CI, 11.0–24.9 months), with a 3-year PFS of 25.7%. Median OS was 35.1 months, with a 3-year survival rate of 27.3%. A review of published SWOG data suggested that OS is determined by the duration of PFS and not the quality of the response [24]. While any form of meaningful response can be associated with clinical benefit in patients with relapsed or refractory MM, the durability of responses with different regimens will be a key determinant in designing the best approach.
In patients with relapsed MM treated with bortezomib and dexamethasone [25], the rates of both neutropenia (13%) and thrombocytopenia (33%) were lower than with regimens containing bortezomib and PEGylated liposomal doxorubicin [26]. Grade 3–4 infections occurred in 33% after bortezomib, doxorubicin, and high-dose dexamethasone [27]. In our study, the incidences of grade 3–4 thrombocytopenia and neutropenia were 35.8% and 7.7%, respectively; however, no patient experienced febrile neutropenia. Relatively lower grade 3–4 infection (15.4%) developed. The risk of infections was markedly lower with low-dose dexamethasone than with a regimen that included both doxorubicin and high-dose dexamethasone. The incidence of grade 3 peripheral neuropathy was 10.3%. Although peripheral neuropathy and grade 3 thrombocytopenia occurred frequently, they were manageable and well tolerated in the majority of the patients.
In conclusion, although the optimal duration of bortezomib followed by thalidomide combination therapy is uncertain, PAD followed by TD sequential combination treatment was very active and well tolerated as a salvage treatment for relapsed or refractory MM. Based upon these promising results, further evaluation with randomized trials is warranted to determine the optimal sequence and combination of available therapies: bortezomib, adriamycin, thalidomide, and dexamethasone.
References
Glasmacher A, Hahn C, Hoffmann F et al (2006) A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol 132:584–593
Richardson PG, Blood E, Mitsiades CS et al (2006) A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood 108:3458–3464
Richardson PG, Sonneveld P, Schuster MW et al (2005) Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352:2487–2498
Bladé J, Vesole DH, Gertz M (2003) High-dose therapy in multiple myeloma. Blood 102(10):3469–3470
Raikumar SV, Fonseca R, Dispenzieri A et al (2000) Thalidomide in the treatment of relapsed multiple myeloma. Mayo Clin Proc 75:897–901
Singhal S, Mehta J, Desikan R et al (1999) Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 341:1565–1571
Rajkumar SV, Hayman S, Gertz MA et al (2002) Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol 20:4319–4323
Weber D, Rankin K, Gavino M et al (2003) Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol 21:16–19
Richardson PG, Barlogie B, Berenson J et al (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348:2609–2617
Richardson PG, Sonneveld P, Schuster M et al (2007) Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood 110:3557–3560
Orlowski RZ, Nagler A, Sonneveld P et al (2007) Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol 25:3892–3901
Faith ED, Noopur R, Teru H et al (2001) Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 98:210–216
Hideshima T, Chauhan D, Shima Y et al (2000) Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood 96:2943–2950
Joan B, Diana S, Donna R et al (1998) Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol 102:1115–1123
Weber DM, Chen C, Niesvizky R et al (2007) Multiple myeloma (009) study investigators. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med 357:2133–2142
Dimopoulos M, Spencer A, Attal M et al (2007) Multiple myeloma (010) study investigators. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 357:2123–2132
Ciolli S, Leoni F, Casini C et al (2008) The addition of liposomal doxorubicin to bortezomib, thalidomide and dexamethasone significantly improves clinical outcome of advanced multiple myeloma. Br J Haematol 141(6):814–819
Attal M, Harousseau JL, Stoppa AM et al (1996) A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med 335:91–97
Child JA, Morgan GJ, Davies FE, Medical Research Council Adult Leukaemia Working Party et al (2003) High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 348:1875–1883
Barlogie B, Tricot G, Anaissie E et al (2006) Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med 354:1021–1030
Rajkumar SV, Dispenzieri A (2005) Evaluation and monitoring of response to therapy in multiple myeloma. Haematologica 90:1305–1308
Palumbo A, Gay F, Bringhen S et al (2008) Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. Ann Oncol 19:1160–1165
Durie BG, Jacobson J, Barlogie B et al (2004) Magnitude of response with myeloma frontline therapy does not predict outcome: importance of time to progression in southwest oncology group chemotherapy trials. J Clin Oncol 22:1857–1863
Jagannath S, Richardson PG, Barlogie B et al (2006) Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to bortezomib alone. Haematologica 91:929–934
Sonneveld P, Hajek R, Nagler A, DOXIL-MMY-3001 Study Investigators et al (2008) Combined pegylated liposomal doxorubicin and bortezomib is highly effective in patients with recurrent or refractory multiple myeloma who received prior thalidomide/lenalidomide therapy. Cancer 112:1529–1537
Oakervee HE, Popat R, Curry N et al (2005) PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol 129:755–762
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Sung Sook Lee and Cheolwon Suh equally contributed to this article.
Rights and permissions
About this article
Cite this article
Lee, S.S., Suh, C., Kim, BS. et al. Bortezomib, doxorubicin, and dexamethasone combination therapy followed by thalidomide and dexamethasone consolidation as a salvage treatment for relapsed or refractory multiple myeloma: analysis of efficacy and safety. Ann Hematol 89, 905–912 (2010). https://doi.org/10.1007/s00277-010-0943-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-010-0943-z