Abstract
The objective of the study was to evaluate the effectiveness of chlorhexidine-impregnated sponges for reducing catheter-related infections of central venous catheters inserted for cancer chemotherapy. The method used was a randomized, prospective, open, controlled clinical study (three-step group sequential analysis protocol). The patients were from two high dependency units at a university hospital undergoing chemotherapy for haematological or oncological malignancies requiring central venous catheters (CVCs) expected to remain in place for at least 5 days. Six hundred and one patients with 9,731 catheterization days were studied between January 2004 and January 2006. Patients admitted for chemotherapy received chlorhexidine and silver sulfadiazine-impregnated triple-lumen CVCs under standardized conditions and were randomized to the groups receiving a chlorhexidine gluconate-impregnated wound dressing or a standard sterile dressing. Daily routine included clinical assessment of the insertion site (swelling, pain, redness), temperature, white blood count and C-reactive protein. Catheters remained in place until they were no longer needed or when a CVC-related infection was suspected. Infection was confirmed with blood cultures via the catheter lumina and peripheral blood cultures according to the time-to-positivity method. Six hundred and one patients were included. The groups were comparable with respect to demographic and clinical data. The incidence of CVC-related infections were 11.3% (34 of 301) and 6.3% (19 of 300) in the control and chlorhexidine-impregnated wound dressing groups, respectively (p = 0.016, relative risk 0.54; confidence interval 0.31–0.94). Especially, catheter-related infections at internal jugular vein insertions could be reduced (p = 0.018). No adverse effects related to the intervention were observed. The use of chlorhexidine-impregnated wound dressings significantly reduced the incidence of CVC-related infections in patients receiving chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients admitted with haematological or oncological diseases frequently require a central venous catheter (CVC) for intra-venous chemotherapy. These individuals are especially at risk as immunosuppression either due to treatment or due to the underlying disease is associated with a higher risk of infection, in particular CVC-related infections [1, 2].
A strict anti-septic regimen for insertion of the catheter and during manipulations of the catheter hubs and the insertion site is crucial to prevent CVC-related infection. In immunocompromised patients, the use of antibiotic-impregnated catheters helps to further reduce CVC-related infections [3, 4]; consequently, we use only impregnated catheters in haematology and oncology patients.
The catheter insertion site is a possible entry point for pathogens causing bloodstream infections. Chlorhexidine gluconate used for skin disinfection during insertion has been demonstrated to prevent CVC-related infections [5]. Therefore, the use of a wound dressing that continuously releases chlorhexidine gluconate at the insertion site has promise for decreasing CVC-related infections. BioPatch® (Ethicon, Norderstedt, Germany) is a chlorhexidine-impregnated sponge (2.5 cm diameter) which can be placed over the CVC insertion site and is covered by a transparent polyurethane dressing. There have been favourable results in paediatric patients undergoing CVC insertion regarding the rates of catheter tip colonization [6, 7]. A randomized study of 33 individuals did not reveal a decrease in catheter tip colonizations and CVC-related infection [8]. Guidelines for the prevention of intra-vascular catheter-related infection [9] do not give a recommendation and regard the use of chlorhexidine sponge dressing an unresolved issue.
There have, as yet, been no studies published to thoroughly examine the anti-infective properties of chlorhexidine gluconate-impregnated wound dressings in the group of patients requiring chemotherapy. Thus, the aim of this study was to investigate the effectiveness of a chlorhexidine dressing in reducing catheter-related infection.
Materials and methods
Patients
The study was planned according to the Declaration of Helsinki and approved by the Hannover Medical School Ethics Committee. All patients gave informed consent to participate in the study.
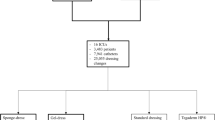
Six hundred and one patients were enrolled into the study. The majority had been admitted for chemotherapy of haematological malignancies. All patients received a triple-lumen CVC impregnated on the exterior surface with silver sulfadiazine–chlorhexidine (Arrogard blu®, Arrow, Erding, Germany). Patients who were expected to have their CVC for less than 5 days were not included. One patient refused to further participate in the study and received the regular, i.e. control group, treatment at his catheter site.
Catheter insertion and management
Experienced board-certified anaesthesiologists inserted chlorhexidine and silver sulfadiazine-impregnated catheters in a special anaesthesiology clinic unit under monitored care either into the internal jugular or the subclavian vein (SCV). All patients received local anaesthetic (lidocaine 2% 5–10 ml); some of the patients requested intra-venous sedation with midazolam (1–5 mg). The catheter insertion followed a strict antiseptic regimen with alcohol spray disinfection of the insertion site (2–4 cm3 of Kodan Tinktur®, Schülke & Mayr, Norderstedt, Germany; skin allowed to dry), sterile gloves and gown, cap and mask for the anaesthesiologist and a sterile adhesive drape. Catheter positions were confirmed via electrocardiogram (ECG) controls (Alphacard®, B. Braun, Melsungen, Germany, or over the marked guidewire of the Arrow set via a custom-made ECG adapter), and the catheters were secured with clips and two skin sutures. A chest X-ray to exclude pneumothorax was only performed when requested for other reasons or following difficult insertions with repeated punctures.
All catheter insertions were scheduled and performed during regular work hours between 8 a.m. and 4 p.m. Before the patients had been sent for from the wards, the study was explained by a certified haematologist, and informed consent was obtained. In the anaesthesia clinic, the patients were randomly assigned to the treatment group or the control group according to computer-generated identification numbers and, thus, received either the impregnated biopatch dressing or a standard sterile transparent wound dressing.
In the wards, daily blood specimens for laboratory assessments were taken including white blood count and C-reactive protein (CRP). The insertion sites were inspected (as far as the patches would allow) and palpated daily by a specialist oncology nurse following the standard operating procedure for CVC care of Hannover Medical School and by a physician (Franke, Zenz). Neither anti-septic ointments nor filters were used. The wound dressings were changed regularly after 1 week or after they had been lifted up for inspection controls.
CVC-related infection
The diagnosis of catheter-related bloodstream infection (CRBSI) is based on clinical assessment, laboratory investigations and an ex vivo culture technique [10]; this culture technique requires the removal of CVC. The time-to-positivity approach offers an in vivo diagnosis.
If clinical signs of tenderness, erythema or swelling around the catheter insertion site or elevated CRP levels suggested infection, especially if the patient showed elevated body temperature (>38.0°C by ear thermometer measurement), blood cultures (BD BACTEC PLUS, Becton & Dickinson, Shannon, Ireland) were taken through each lumen of the CVC and also via a peripheral venipuncture after skin disinfection. Blood cultures were immediately transferred to the incubator and kept at 37°C (5% CO2). According to this time-to-positivity method, a CVC-related infection was confirmed, if one of the catheter-drawn cultures became positive at least 2 h earlier than the peripheral cultures [11].
Thus, the diagnosis of a CRBSI, according to the Healthcare Infection Control Practices Advisory Committee definitions [9], was made with a proven infection with the time-to-positivity method, and clinical symptoms (fever, swelling, and/or hypotension) for which no other source than the catheter was identified.
In the individuals with confirmed CVC-related infection, the attending haematologist initiated a change of the CVC; in addition, all the removed catheter tips were cultured. CVC change was always carried out with a fresh puncture, and catheters were not changed over guidewires. Catheters were removed when no longer needed for treatment or when the patients were discharged home. Most of these catheters were sent for culture when discontinued.
Sample size calculation
Based on previous data, the anticipated incidence rate of CVC-related infection was conservatively estimated at 6.0% in the control group. A one-sided group sequential plan was designed with two interim analyses to allow for early cessation of the study, if the null hypothesis was rejected. The boundary shape parameters according to Pampallona and Tsiatis were chosen as 0.3 for rejection of the null hypothesis (H0). With an overall significance level of α = 0.05 and a power of 80%, a maximum of 707 patients per group had to be planned for the study. The nominal critical values on the z-scale for the three stages were 1.776, 1.926 and 2.212 for rejection of H0, corresponding to one-sided nominal significance levels of 0.0135, 0.0271 and 0.0379. The z-scale boundaries for the acceptance of H0 were −0.068, 1.063 and 1.776. The sequential plan was calculated by using the software EAST 3.0 (Cytel Software 2003, Cambridge, NY, USA). Data collection was done with an Excel® database (Microsoft, Redmond, WA, USA). The primary endpoint was the rate of CVC-related infection in each group. Incidence rates of CVC-related infections are reported per 1,000 catheter days.
A number-needed-to-treat calculation was performed based on the data of this study.
Results
Six hundred and one individual CVCs were examined in two groups over a time of 9,731 days. The groups were comparable with respect to their demographic data like gender, age and underlying diseases (Table 1). CVC insertion duration and neutropenia days were comparable: The mean durations of CVC insertion were 16.6 (treatment) and 15.8 days (control); the patients had a mean neutropenia period of 7.5 (treatment) and 6.9 days (control).
With the exception of infection, no complications of CVC insertion were observed.
No patient had to be excluded from the study as a consequence of allergic reactions to the chlorhexidine-impregnated foam.
After the first interim analysis, the boundaries for early stopping were not reached. The second interim analysis revealed statistically significant differences. In all patients, a number of 53 CVC-related infections were found. In the treatment group (n = 300; 4,986 days), there were 19 cases of confirmed CVC-related infection, and in the control group (n = 301; 4,795 days), there were 34 cases. The one-sided p value of 0.0160 was lower than the nominal α of 0.0271 (Table 2); thus, the study could be stopped with the rejection of H0. Overall, the rate of CVC-related infections was 46% less in the study group than in the control group (relative risk of 0.54; confidence interval [CI] 0.31–0.94). Regarding insertion sites separately, there was a drop in CRBSI at the internal jugular vein (IJV) CVCs from 30 to 14 cases, as compared to the SCV. The C 2 test of each site did not show statistical significance.
The bacteria isolated from the catheter tips to cause CVC-related infections were mainly Staphylococcus epidermidis in both the treatment group (11 of 19 cases [57.9%]) and in the control group (22 of 34 cases, i.e. 64.7%).
Given the setting of our patients with haematological and oncological diagnoses, a number-needed-to-treat of 19.2 would result, meaning 19.2 patients were to be treated to prevent one episode of CVC-related infection.
Discussion
The influence of chlorhexidine-impregnated sponges on the probable reduction of infections related to CVC in haematological–oncological patients has not been examined previously. In this prospective randomized clinical trial with chlorhexidine-impregnated sponges for dressing of CVC insertion sites, a 46% reduction in CVC-related infections was achieved. External colonization is a major mechanism of CVC-related infections. The results are remarkable as various measures such as standardized insertion procedure, the use of impregnated catheters and consequent CVC care and surveillance had been established successfully prior to the study [12]. The sponges were changed once a week; the mean catheterization duration was 15.8 (control) and 16.6 days (study group) so that the continuous effect of the sponges was maintained beyond the 1-week release of the anti-infective chlorhexidine–silver sulfadiazine [13] of the CVC. However, the overall rate of infections which was higher than in the calculation may have been influenced by the long durations of catheterization, which put the patients at higher risk despite all precautions taken.
Levy et al. [7] investigated 166 infants and children between 0 and 18 years from a cardiac intensive care unit. The mean catheterization duration was given with 4.5 days, and follow-up data were missing. There was no treatment recommendation given. Seven hundred and five neonates with a mean CVC duration of 17 days were analysed by Garland [6]; they found 12 infections in 335 individuals in the treatment group and 11 infections in 370 individuals (odds ratio [OR] 1.21) in the control group.
In 1998, an investigation of exit-site and bloodstream infections was presented with 33 analysed cases of critically ill patients [8]. However, the data presented were not complete so that no recommendation to use the impregnated dressing was given. In a population of 50 surgical patients, Hanazaki et al. [14] found a marked reduction in exit-site colonizations; unfortunately, the duration of catheterization was not given, and rates of CRI were not investigated in that study. Maki et al. [15] presented a number of 1,401 catheters with and without impregnated dressings. The effects of impregnated dressings were promising with respect to exit-site colonizations and CVC-related infection; detailed information on catheter types and durations and follow-up data have been missing so far. Chambers et al. reported a reduction in exit-site or combined exit-site/tunnel infections from 43% to 10% with the use of chlorhexidine-impregnated dressings [16].
In a recent meta-analysis [17], eight studies on epidural catheters and CVCs in paediatric and adult patients were analysed. Chlorhexidine impregnation was described as effective in the reduction in vascular and epidural catheter access colonization and, at least, associated with the reduction in CVC-related infections. The authors had demanded the necessity of larger randomized controlled trials to confirm the hypothesis that impregnated wound dressings help reduce the incidence of CVC-related infections.
If one adds our results to those studies of CVC-related infections to the meta-analysis of Ho and Litton [17], this will give a significant summarized OR of 0.53 (95% CI 0.43–0.64).
We calculated a number-needed-to-treat of 19.2, which means 19.2 patients have to be treated to prevent one episode of CVC-related infection. The treatment over 16 days usually required three patches at a price of 6€ each. To prevent one episode of infection, the expenses would therefore be 3 (sponges) × 6€ (per sponge) × 19 (NNT) = 342€, approximately.
In a recent analysis with intensive care patients, attributable costs of 11,971$ were determined per CVC-related infection [18]. Compared to these costs, the use of impregnated dressings appeared cost effective, even when taking the different patient population into account.
In our investigation, we had comparable patient groups regarding demographic data, diagnoses, durations of catheterization and neutropaenia. In the treatment group, an incidence of 19 CVC-related infections in 300 individuals (6.3%) was determined, and in the control group, there were 34 cases of CVC-related infections in 301 patients (11.3%). Those patients treated with BioPatch® had a significantly lower rate of CRI (p = 0.016). This could be achieved in a highly elaborated setting with a strictly sterile puncture technique and impregnated catheters, the use of which being a consequence from a former study of our group [3].
There was a marked reduction in infections caused by S. epidermidis (22 vs. 11 infections). IJV catheters are said to have a higher risk of infection than SCV catheters, although this has not been proven in randomized controlled trials [19]. In this study, the reduction in CRBSI was statistically significant (p = 0.018 with χ 2 for IJV). Regarding the risk of severe complications of SCV puncture like pneumothorax and bleeding, puncture of the IJV was preferred. All punctures of both sites were carried out without complications.
All patients from the study group tolerated the chlorhexidine-impregnated foam material well, and no patient had to be excluded because of allergic reaction [20] to chlorhexidine as described after use in urethral gels [21]. There was no suspicion of bacterial resistance to chlorhexidine dressings as reported in Pseudomonas spp. [22].
The study presented was conducted as a randomized, controlled prospective trial. The patients received the CVCs by experienced anaesthesiologists; there were continuous catheter care and infection surveillance in the wards. A high number of patients was calculated to have significant results. Being not blinded may be considered a limitation: Unfortunately, non-impregnated patches as ‘dummies’ which could have offered blinded conditions could not be provided by the manufacturer. However, there have in fact been both high expectations and strong skepticism among the physicians and nurses who assessed the patients. By having nurses who were not involved in the study assess the insertion sites and microbiologists unaware of the patients’ group assignments, we aimed to reduce the influence of the design not being blinded.
In conclusion, chlorhexidine-impregnated dressings significantly decrease the risk of CRI in patients at high risk of infection. There is a significant reduction in IJV insertion site infections.
References
Dettenkofer M, Wenzler-Rottele S, Babikir R et al (2005) Surveillance of nosocomial sepsis and pneumonia in patients with a bone marrow or peripheral blood stem cell transplant: a multicenter project. Clin Infect Dis 40(7):926–931
Hanna H, Afif M, Alakech B et al (2004) Central venous catheter-related bacteremia due to gram-negative bacilli: significance of catheter removal in preventing relapse. Infect Control Hosp Epidemiol 25(8):646–469
Jaeger K, Zenz S, Jüttner B et al (2005) Reduction of catheter-related infections in neutropenic patients: a prospective controlled randomized trial using a chlorhexidine and silver sulfadiazine-impregnated central venous catheter. Ann Hematol 84(4):258–262
Raad I, Hanna HA, Alakech B et al (2004) Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Intern Med 140(1):18–25
Maki DG, Ringer M, Alvarado CJ (1991) Prospective randomised trial of povidone–iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet 338(8763):339–343
Garland JS, Alex CP, Mueller CD et al (2001) A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infection in neonates. Pediatrics 107(6):1431–1416
Levy I, Katz J, Solter E et al (2005) Chlorhexidine-impregnated dressing for prevention of colonization of central-venous catheters in infants and children: a randomized controlled study. Pediatr Inf Dis 24(8):676–679
Roberts B, Cheung D (1998) Biopatch—a new concept in antimicrobial dressings for invasive devices. Aust Crit Care 11(1):16–19
O’Grady NP, Alexander M, Dellinger EP , Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Raad I, Randolph A (2002) Guidelines fort he prevention of intravascular catheter-related infections. Morb Mortal Wkly Rep Recomm Rep. 52(RR-10):1–29
Maki DG, Weise CE, Sarafin HW (1977) A semiquantitative method for identifying intravenous-catheter-related infections. N Engl J Med 296(23):1305–1309
Blot F, Schmidt E, Nitenberg G et al (1998) Earlier positivity of central-venous- versus peripheral-blood cultures is highly predictive of catheter-related sepsis. J Clin Microbiol 36(1):105–109
Pronovost P, Needham D, Berenholtz S et al (2006) An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 355(26):2725–2732
Crnich CJ, Maki DG (2002) The promise of novel technology for the prevention of intravascular device-related bloodstream infection. I. Pathogenesis and short-term devices. Clin Infect Dis 34:1232–1242
Hanazaki K, Shingu K, Adachi W et al (1999) Chlorhexidine dressing for reduction in microbial colonization of the skin with central venous catheters: a prospective randomized controlled trial. J Hosp Infect 42:165–168
Maki DG, Mermel LA, Kluger D et al (2000) The efficacy of a chlorhexidine-impregnated sponge (Biopatch) for the prevention of intravascular catheter-related infection: s prospective randomized, controlled multicenter study (abstract). In: Proceedings of the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington DC, Sept. 2000, Abstract 1430, p 422
Chambers ST, Sanders J, Patton WN et al (2005) Reduction of exit-site infections in tunnelled intravascular catheters among neutropenic patients by sustained-release chlorhexidine dressings: results from a prospective randomized controlled trial. J Hosp Infect 61:53–61
Ho K, Litton E (2006) Use of chlorhexidine-impregnated dressing to prevent vascular and epidural catheter colonization and infection: a meta-analysis. J Antimicrob Chemother 58:281–287
Warren DK, Quadir WW, Hollenbaek CS et al (2006) Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit Care Med 34(8):2084–2089
Rüsch S, Walder B, Tramèr MR (2002) Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med 30(2):454–460
Kluger M (2003) Anaphylaxis to chlorhexidine-impregnated central venous catheter. Anaesth Intensive Care 31(6):697–698
Jayathillake A, Mason DF, Broome K (2003) Allergy to chlorhexidine gluconate in urethral gel: report of four cases and review of the literature. Urology 61(4):837
Tattawasart U, Maillard JY, Furr JR et al (1999) Development of resistance to chlorhexidine diacetate and cetylpyridinium chloride in Pseudomonas stutzeri and changes in antibiotic susceptibility. J Hosp Infect 42(3):219–229
Acknowledgements
The authors are especially indebted to the nurses at the Department of Haematology, Haemostasis, Oncology, and Stem Cell Transplantation at Hannover Medical School. The authors thank Christian Apfel M.D. for critical review of the manuscript.
Funding and conflict of interest statement
The study was carried out without any external funding. Part of the results of this study was presented as an abstract at the Annual Meeting of the European Society of Anaesthesiologists (ESA) in Madrid in June 2006. H.R. and M.F. received travel grants by Ethicon/Johnson & Johnson.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruschulte, H., Franke, M., Gastmeier, P. et al. Prevention of central venous catheter related infections with chlorhexidine gluconate impregnated wound dressings: a randomized controlled trial. Ann Hematol 88, 267–272 (2009). https://doi.org/10.1007/s00277-008-0568-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-008-0568-7