Abstract
Purpose. Arterial supply of thalamus is complex and highly variable. In particular, the distribution pattern of thalamoperforating arteries received more attention some decades ago than in recent years. Methods. We are presenting the case of a 46-year-old patient with wake-up drowsiness, complex oculomotor disorder and dysarthria. He was investigated in the acute phase using non-contrast brain Computed Tomography (NCCT), CT Angiography (CTA), and in the following days Digital Subtraction Angiography (DSA) was performed Results. The NCCT showed a subacute ischemic stroke in the right anterior thalamus and rostral midbrain with normal findings on CTA. DSA imaged a variant of thalamic supply (Percheron type III), constituted by perforating branches arising from an artery bridging the P1 segments of both Posterior Cerebral Arteries (PCAs). Results. The thalamus has a complex and variable arterial supply, mainly in the pattern of paramedian thalamic-mesencephalic perforating arteries. The most reported variant is Percheron type IIb and supplies the paramedian thalami and the rostral midbrain. Type IIb occlusion usually causes a bilateral paramedian thalamic stroke, but rostral midbrain and anterior thalamus are involved in 57% and 19% cases. The rarer Type III variant probably prevented the bilateral extension of infarction and involved the territory of tuberothalamic and paramedian perforating arteries. Conclusions. Currently, DSA allows directly imaging variants in thalamic vascularization and better understanding the stroke mechanisms. In particular, in the presented case, a medium-sized vessel occlusion rather than a small vessel occlusion mechanism might be raised, leading to a different diagnostic pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thalamus has a complex and highly variable arterial supply and this feature has been detailed in both neuroanatomical and neuroimaging studies [2, 16, 24]. The posterior portion of the circle of Willis is formed at earlier stages of the embryo, when the fetal PCA turns into posterior communicating artery (PcomA) and branches from the fetal PCA fuse medially to form the distal end of the BA, while the adult PCA connects with the BA [22]. The embryology and variations of PCA branches, and in particular the thalamoperforating arteries, received less attention than other intracranial arteries [31], being investigated mainly for neurosurgical approaches [5, 9, 19]. The lack of detailed information about some PCA-related embryological issues was attributed to the relatively late stage when its development occurs, preventing to have serial sections of a too large fetus [20]. The PCA is embryologically a diencephalon-mesencephalic vessel, as pointed out by Padget [22, 23], Lazorthes [13], and Lasjaunias [12]. At the Padget stage IV and V [22, 23] the P2, P3, P4 PCA segments were formed by the main trunk of the Posterior Choroidal Artery (PChoA) with its telencephalic branch; at Padget stage VII [22, 23], the PCA gathers the telencephalic supply and, from stage VI, the mesencephalic-PCA territory is supplied progressively more by the vertebrobasilar system [1, 17, 22, 23, 26]. Padget [22, 23] and Moffat [20] did not describe the embryological development of the perforating branches of the PCA, limiting the observation to say that from Padget stage 5 until the end of the development, the perforators branches originate variably and inconstantly mainly from P1 and P2 segments [23, 31]. Lasjaunias [12] described a collicular group of arteries originating from the distal portion of the caudal division of the internal carotid artery (ICA) at the P1 PCA-basilar artery (BA) junction including the superior cerebellar artery (SCA). These collicular or long circumflex arteries can be considered embryologically derived from the mesence¬phalic artery of Moffat’s embryo [12, 20, 31]. The interaction between the original perforators present in the embryo and the neuro-embryogenesis of diencephalic structures is not deeply known and the vascular territories are highly variable [2, 4]. The main anatomical variants of P1 PCA branches were described by Gustave Percheron, from which it was named the most commonly known variant (artery of Percheron or AOP), a single arterial trunk arising from the P1 PCA and supplying the paramedian territory of the bilateral thalami and the rostral midbrain [24]. AOP is the variant Iib, but several other variants were described [2, 4, 16, 22], although neglected, and they might also be relevant in neurovascular practice, as highlighted by our case, showing a type III variant of the PCA. Actually, DSA allows to directly imaging perforating vessels [9, 15] and to assess the pattern of thalamoperforating arteries other than AOP. In fact, although thalamic stroke is often a challenge for the reasoning on the etiological diagnosis, the topography of the ischemia on neuroimaging [3] can be a useful clue to infer the pattern of vascularization and guide the diagnostic pathway, helping in differentiating small vessel occlusion mechanisms from medium-sized vessel occlusions.
Methods
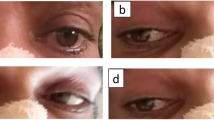
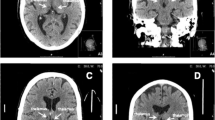
We report the case of a 46-year-old man with a history of migraine without aura who went to the Emergency Department (ED) because of the occurrence of drowsiness and diplopia at wake-up. Neurological examination in the ED showed a complex oculomotor disorder, torsional upward nystagmus, dysarthria and a mild left hemiataxia (National Institute of Health Stroke Scale – NIHSS - score 3). Non-contrast CT (NCCT) found a subacute ischemia in the right anterior thalamus and CT angiography (CTA) did not show abnormalities. The patient was admitted in Stroke Unit and started antiplatelet therapy with a quick improvement of both oculomotor disorder and left hemiataxia with 24 h NIHSS score 1. On day two from the ED admittance a brain Magnetic Resonance Imaging (MRI) was performed (Fig. 1a), showing an acute ischemic lesion involving the paramedian zone of the upper midbrain, the middle and anterior portion of the thalamus on the right side, similar to a single branch of a V in the coronal plane (Fig. 1b). These results could suggested a small vessel disease (SVD) related stroke. The MR Angiography (MRA) was unremarkable (Fig. 1c). The topography of the ischemia suggested the simultaneous involvement of different territories: (I) the rostral midbrain and the paramedian thalamus, usually supplied by perforating arteries originating from the P1 segment of the posterior cerebral artery (PCA); (II) the anterior thalamus, usually supplied by the tuberothalamic arteries, frequently reported as originating from the PComA. However, since the involvement of multiple perforating arteries is uncommon in SVD related stroke and since parent arteries were normal on CTA and MRA, we suspected the occlusion of a single medium-sized artery. Therefore, a DSA was performed (Fig. 1d-g, movie 1), revealing an arterial arcade linking both P1 segments, branching off in thalamoperforating trunks, which indicate a type III variant of the Percheron’s classification [24]. In addition, contrast extravasation in the territory of affected thalamoperforating arteries raising from the arcade was evident on DSA, corresponding to blood brain barrier damage related to the subacute ischemia in the related territory. At the time of DSA, the arterial arcade joining right and left P1 PCA was patent, so we may speculate about a previous occlusion of a portion or limb of the arcade, reducing the perfusion in the terminal territory of the thalamoperforating arteries supplying the affected territory and raising from the arcade.
(a) Brain MRI (Magnetic Resonance Imaging), axial Fluid Attenuating Inversion Recovery (FLAIR) sequences, showing, in a caudal-cranial line, an hyperdense signal corresponding to the subacute ischemic lesion involving the rostral midbrain, paramedian thalamus and anterior thalamus on the right side. (b) Brain MRI, coronal FLAIR sequence, showing the infarct shaped as single branch of the V sign described in type IIB variant stroke [2]. (c) Intracranial MRA (time of flight) in a coronal plane reconstructed using MIP/MPR. (d-h) Digital Subtraction Angiography (DSA) of top of the basilar artery and PCA from left vertebral artery injection. The asterisk (*) points to the arcade connecting right and left P1 PCA in a direct acquisition image (e), tridimensional reconstruction of rotational angiograhy (3D-RA) (f) and MIP/MPR image (g). The red arrow points to the two main trunks of thalamoperforating arteries arising from the arcade (detail of figure g in the square h) and the white arrowhead points to the contrast extravasation due to the blood brain barrier damage in the location of the subacute infarction
Discussion
The embryology and variations of PCA and its branches received less attention than other intracranial arteries [7, 8], in particular, deep cerebral perforators [30] which are a relatively neglected issue and investigated mainly for neurosurgical approaches [5, 9, 19]. The lack of detailed information about some PCA-related embryological issues was attributed to the relatively late stage when its development occurs, preventing to have serial sections of a too large fetus [20]. Both the adult configuration [6] and the precise embryological development of the perforating branches of the PCA remains poorly known and, as a general rule, perforators deriving embryologically from the same vessel in the adult configuration present a large variability in the assignment to a perforator group [30]. Padget [22, 23] and Moffat [20] did not describe the embryological development of the perforating branches of the PCA, limiting the observation to say that from Padget stage 5 until the end of the development, the perforators branches originate variably and inconstantly mainly from P1 and P2 segments [23, 31]. Lasjaunias [12] described a collicular group of arteries originating from the distal portion of the caudal division of the internal carotid artery (ICA) at the P1 PCA-basilar artery (BA) junction including the superior cerebellar artery, (SCA). These collicular or long circumflex arteries can be considered embryologically derived from the mesencephalic artery of Moffat’s embryo [12, 20, 31]. The interaction between the original perforators present in the embryo and the neuro-embryogenesis of diencephalic structures is not deeply known.
The P1 PCA gives origin to direct perforating arteries, globally called thalamoperforating arteries, supplying the anterior and part of posterior thalamus, hypothalamus, subthalamus, substantia nigra, red nucleus, oculomotor nerve, mesencephalic reticular formation, pretectum, rostromedial floor of fourth ventricle, and posterior portion of the internal capsule [18, 30, 31]. The mean number of direct thalamoperforating arteries arising from each P1 is 2.7 according with Zeal and Rhoton [30] and 3.1 according with Djujelic [5], but in 2% of cases they might be absent [17] on one side and originating from the contralateral P1 segment [12]. They origin mostly from the middle-third of P1 [5, 9], but Kaya described in one specimen the thalamoperforating arteries starting out from a fenestrating branch between the right and the left P1 segment [9], similarly to the case we are presenting. A further point of variability in the anatomy and pattern of supply of thalamoperforating arteries is the existence of anastomoses between them, well described by Marinkovic [19] in the 79% of the examined cases. These anastomotic channels had a main diameter of 148 µmm and an average length of 3.3 mm [9] and a subtle and detailed classification of these anastomoses in five groups was proposed [19].
In this context, it is not surprising to consider the territory fed by the paramedian thalamic-mesencephalic perforating branches as the most variable one [2, 4]. Among the several variations, Gustave Percheron in 1973 described in a systematic classification these anatomical variants (Fig. 2). The most commonly known variant has been called “artery of Percheron” (AOP) and it is a single arterial trunk arising from the P1 PCA and supplying the paramedian territory of the bilateral thalami and the rostral midbrain [24]. The embryological basis of AOP and the other variations of the P1 PCA branches is not well understood, but two different hypotheses were proposed: (I) an asymmetrical fusion of longitudinal neural arteries (LNA) at the BA tip [10, 21]; (II) an incomplete regression of primitive trigeminal arteries at the vertebrobasilar junction [28]. However, the AOP naming is a misnomer, because Percheron described four different variants and the one known as AOP is better identified as the variant IIb. The attention raised by the variant IIB led to substantially neglect the other variants [2, 4, 16, 22], but they might also be relevant in neurovascular practice, as outlined by our case, showing a different variant (type III in the classification of Percheron) [24], i.e. an arcade of perforating branches arising from an artery bridging the P1 segments of both PCAs [16, 19]. This variation could be considered as a fenestration of the BA tip resulting from an incomplete fusion of the LNA [27], accordingly with the hypothesis of Lasjaunias [12], who described thalamoperforating arteries as a collicular group of arteries originating from the distal portion of the caudal division of the ICA. Then, type III of Percheron’s artery may result from an incorporation of collicular arteries of Lasjaunias into a fenestrated BA tip during the fusion of the caudal division of the ICA with the vertebrobasilar system.
Variants of the paramedian thalamic and midbrain supply according to Percheron [24]. BA: basilar artery; M: midbrain; PCA: posterior cerebral artery; Th: thalamus
An occlusion of the AOP usually causes an ischemic lesion involving the paramedian thalamus bilaterally and sometimes the rostral midbrain (57%) and/or the anterior thalamus (19%) in the territory usually supplied by tuberothalamic perforating arteries [14]. Indeed, the classical appearance of the AOP infarction, involving bilaterally both upper midbrain and paramedian thalamus, is rare in the published case series of AOP infarctions and defines a “V” sign in coronal plane.
While in the past, the possibility of defining the supplying pattern of the anterior thalamo-perforating arteries derived from cadaveric dissection studies [24], actually DSA, in particular with three-dimensional rotational angiography (3D-RA) and Minimum Intensity Projection/MultiPlanar Reconstruction (MIP/MPR) allows to directly imaging perforating vessels [9, 15]. In our case, the infarct’s location suggested a variant of the thalamic supply different from an AOP and the noninvasive neuroimaging techniques were not able to answer this question. DSA may directly image not only AOP but also the other variants of the thalamic supply. The type III variation presented in our case (Figs. 1d-g and 2), defined as an artery bridging the P1 segments of both PCAs and giving origin to thalamoperforating arteries [16, 19, 24] was reported even rarely than AOP [14], but a single anatomical report on cadaver dissection found an anastomosis connecting the right and left P1 PCA in 10% of cases [11, cited by 25]. Then, it received less attention in the literature and its impact on the localization pattern of thalamus and midbrain infarction is less know. In the presented case an ischemic lesion involving the rostral midbrain, the paramedian thalamic and the anterior thalamic territory on the right side is showed with a neuroimaging appearance as single branch of the V sign described in AOP infarction [2, 4]. DSA with 3D-RA allowed to image this variant and the anastomotic arcade between the P1 segments of the PCA on both sides with the main trunks of the perforating arteries are defined as arising from this arcade (Fig. 2). Moreover, it is possible to hypothesize that the presence of a type III variant prevented the bilateral extension of the thalamo-mesencephalic infarction during the acute phase, when the right branch of the anastomotic arcade is supposed to be occluded. At the time of the DSA the arcade was patent, so a spontaneous recanalization may be proposed as hypothesis. The involvement of the tuberothalamic arteries (or anterior polar artery [24]) territory might be because paramedian perforating arteries supply it, as in AOP variant. Although the tuberothalamic arteries are usually branches of the PComA, their absence is not rare (one-third of individuals) [3, 9, 14]. In this case, the paramedian arteries or, even more commonly, the AOP, supply the anterior thalamic territory [15]. In our case, the type III variant supplied unilaterally the territory usually covered by AOP. In AOP infarction an asymmetric topography of the infarction among the two sides has been frequently reported (nearby to 90%) [29], so multiple combinations of anatomic variants [9, 17] and variant territories [3, 14, 29] may be supposed. However, we cannot exclude the possibility of a patent arcade with an occlusion of multiple ipsilateral thalamoperforating arteries arising from the arc. Both hypotheses are possible, because the infarct affected the territory of the ipsilateral thalamoperforating arteries downstream of its origin from the arch and it is not possible to distinguish between hypoperfusion due to the occlusion of the arcade and hypoperfusion due to the occlusion of the perforating branches arising from the arcade. In general, SVD is associated to the occlusion of a single perforating artery, so, being on our case the final infarct location more probably related to more than one perforating vessel territory, a transient occlusion of the right limb of the arcade between the two P1 PCA can be considered more probable in our case, although not demonstrated.
Therefore, in the presented case the identification of a Type III Percheron variant allowed to consider the hypothesis of a medium vessel rather a small vessel occlusion as the main occlusive pattern of our patient, guiding the diagnostic pathway towards the identification of an embolic source (not found) and the exclusion of a P1 PCA involvement (e.g. an intracranial dissection).
Conclusions
The use of increasingly efficient angiographic techniques could help to define the anatomical and hemodynamic factors involved in thalamic stroke. Finally, the knowledge and identification of the above-described variants might be of utmost relevance in acute stroke treatment, because variants IIb and III defined the occlusion of medium-sized branches and not of single or multiple perforating branches.
Data availability
No datasets were generated or analysed during the current study.
References
Bertulli L, Robert T (2021) Embryological development of the human cranio-facial arterial system: a pictorial review. Surg Radiol Anat 43:961–973. https://doi.org/10.1007/S00276-021-02684-Y
Bordes S, Werner C, Mathkour M, McCormack E, Iwanaga J, Loukas M, Lammle M, Dumont AS, Tubbs RS (2020) Arterial supply of the Thalamus: a Comprehensive Review. World Neurosurg 137:310–318. https://doi.org/10.1016/j.wneu.2020.01.237
Carrera E, Michel P, Bogousslavsky J (2004) Anteromedian, central, and posterolateral infarcts of the thalamus: three variant types. Stroke 35:2826–2831. https://doi.org/10.1161/01.STR.0000147039.49252.2f
Cosson A, Tatu L, Vuillier F, Parratte B, Diop M, Monnier G (2003) Arterial vascularization of the human thalamus: extra-parenchymal arterial groups. Surg Radiol Anat 25:408–415. https://doi.org/10.1007/s00276-003-0153-7
Djulejić V, Marinković S, Milić V, Georgievski B, Rašić M, Aksić M, Puškaš L (2015) Common features of the cerebral perforating arteries and their clinical significance. Acta Neurochir 157:743–754
Dobrocky T, Matzinger M, Piechowiak EI, Kaesmacher J, Pilgram-Pastor S, Goldberg J, Bervini D, Klail T, Pereira VM, Z’Graggen W, Raabe A, Mordasini P, Gralla J (2023) Benefit of advanced 3D DSA and MRI/CT Fusion in Neurovascular Pathology. Clin Neuroradiol 33(3):669–676. https://doi.org/10.1007/s00062-022-01260-0
Endo H, Ono H, Nakamura H (2023) Complete duplication of the posterior cerebral artery. Surg Radiol Anat 45(4):359–361. https://doi.org/10.1007/s00276-023-03095-x
Endo H, Ishikawa K, Ono H, Honjo K, Nakamura H (2024) Replaced posterior cerebral artery. Surg Radiol Anat 46(3):299–302. https://doi.org/10.1007/s00276-023-03294-6
Kaya AH, Dagcinar A, Ulu MO, Topal A, Bayri Y, Ulus A, Kopuz C, Sam B (2010) The perforating branches of the P1 segment of the posterior cerebral artery. J Clin Neurosci 17(1):80–84. https://doi.org/10.1016/j.jocn.2009.03.046
Klostranec JM, Krings T (2022) Cerebral neurovascular embryology, anatomic variations, and congenital brain arteriovenous lesions. J Neurointerv Surg 14(9):910–919. https://doi.org/10.1136/neurintsurg-2021-018607
Lang J, Brunner FX (1978) Rami Diencephalici inferiores anteriores, inferiores, inferiores posteriores und inferiores laterales posteriores–Anzahl, Durchmesser, Ursprungs- Und Verlaufsvariationen [Inferior anterior, inferior, inferior posterior and inferior lateral posterior diencephalic branches–number, diameter, origin and flow variations]. Verh Anat Ges 72:429–431
Lasjaunias P, Berenstein A (1987) Surgical neuroangiography. Springer, Berlin, Heidelberg
Lazorthes G (1961) Vascularisation Et circulation cérébrales. Masson, Paris
Lazzaro NA, Wright B, Castillo M, Fischbein NJ, Glastonbury CM, Hildenbrand PG, Wiggins RH, Quigley EP, Osborn AG (2010) Artery of Percheron infarction: imaging patterns and clinical spectrum. AJNR Am J Neuroradiol 31:1283–1289. https://doi.org/10.3174/ajnr.A2044
Li X, Agarwal N, Hansberry DR, Prestigiacomo CJ, Gandhi CD (2015) Contemporary therapeutic strategies for occlusion of the artery of Percheron: a review of the literature. J Neurointerv Surg 7(2):95–98. https://doi.org/10.1136/neurintsurg-2013-010913
Li S, Kumar Y, Gupta N, Abdelbaki A, Sahwney H, Kumar A, Mangla M, Mangla R (2018) Clinical and neuroimaging findings in thalamic territory infarctions: a review. J Neuroimaging 28(4):343–349. https://doi.org/10.1111/jon.12503
Marchi F, Bonasia S, Robert T (2023) Embryology and anatomy of the posterior cerebral artery. In: Robert T, Bonasia S, Bojanowski MW (eds) Anatomy of cranial arteries, Embryology and variants. Springer, Cham. https://doi.org/10.1007/978-3-031-32913-5_14
Marchi F, Bonasia S, Robert T (2023) Perforating branches of the posterior cerebral artery. In: Robert T, Bonasia S, Bojanowski MW (eds) Anatomy of cranial arteries, Embryology and variants. Springer, Cham. https://doi.org/10.1007/978-3-031-32913-5_15
Marinkovic SV, Milisavljevic MM, Kovacevic MS (1986) Anastomoses among the thalamoperforating branches of the posterior cerebral artery. Arch Neurol 43:811–814. https://doi.org/10.1001/archneur.1986.00520080053020
Moffat DB (1961) The development of the posterior cerebral artery. J Anat 95:485–494
Ota T (2022) Functional arterial anatomy of the brain. Stroke: Vascular Interventional Neurol 2(5):e000446
Padget DH (1945) The circle of Willis: its embryology and anatomy. In: Dandy WE (ed) Intracranial arterial aneurysm. Comstock, New York, pp 74–85
Padget DH (1948) The development of the cranial arteries. Contrib Embryol 32:207–261
Percheron G (1973) The anatomy of the arterial supply of the human thalamus and its use for the interpretation of the thalamic vascular pathology. Z Neurol 205:1–13
Ratanpara L, Xalxo N, Chauhan PR, Mehra S (2024) Artery of Percheron, an uncommon variant of posterior cerebral circulation: a Case Report. Cureus 16(3):e57266. https://doi.org/10.7759/cureus.57266
Raybaud C (2010) Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg Clin N Am 21:399–426. https://doi.org/10.1016/j.nec.2010.03.011
Sogawa K, Kikuchi Y, O’uchi T, Tanaka M, Inoue T (2013) Fenestrations of the basilar artery demonstrated on magnetic resonance angiograms: an analysis of 212 cases. Interv Neuroradiol 19(4):461–465. https://doi.org/10.1177/159101991301900409
Sparacia G, Agnello F, Midiri M, Iaia A (2018) A rare case of ruptured aneurysm of the paramedian artery of Percheron. Interv Neuroradiol 24(5):509–512. https://doi.org/10.1177/1591019918775953
Taydas O, Ogul Y, Ogul H (2022) Association with clinic risk factors of Percheron artery infarction and magnetic resonance imaging involvement patterns. Acta Neurol Belg 122:411–415. https://doi.org/10.1007/s13760-021-01697-z
Vogels V, Dammers R, Van Bilsen M, Volovici V (2021) Deep cerebral perforators: anatomical distribution and clinical symptoms: an overview. Stroke 52:E660–E674
Zeal AA, Rhoton AL (1978) Microsurgical anatomy of the posterior cerebral artery. J Neurosurg 48:534–559. https://doi.org/10.3171/jns.1978.48.4.0534
Acknowledgements
Not applicable.
Funding
The authors report no targeted funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MZ, MN and RP. The first draft of the manuscript was written by MZ and revised by FV. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Research ethics and informed consent
An Ethical Board approval is not applicable for this case and a consent-to-disclose form has been acquired by the patient.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Movie 1 (supplemental material) shows the rotational view of the 3D-RA
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zedde, M., Grisendi, I., Assenza, F. et al. Type III Percheron’s variant in thalamic-mesencephalic infarction: the unexpected anastomosis. Surg Radiol Anat (2024). https://doi.org/10.1007/s00276-024-03472-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00276-024-03472-0