Abstract
Introduction
Lateral pharyngotomy (LP) is a surgical procedure that allows exposure to tumors of the pharynx and supraglottic larynx. This study was undertaken to: (1) propose a classification system of LP used in exposing various sites of the oropharynx, supraglottis, and hypopharynx. (2) Describe the structures visible with each category of LP.
Materials and methods
Five tissue-fixed human cadavers from our gross anatomy laboratory were dissected in a manner similar to surgical lateral pharyngotomy. After exposure of the neurovascular structures of the anterior compartment of the neck and laryngeal framework, traditional pharyngotomy was performed with entry between the hypoglossal nerve cephalically and the superior laryngeal nerve caudally (traditional LP). Progressively increased exposure was created by division of adjacent structures. The ability to visualize certain structures (epiglottis, ipsilateral and contralateral base of tongue, postcricoid area, arytenoids, uvula, soft palate, and vallecula) through the pharyngotomy was recorded.
Results
The epiglottis and ipsilateral tongue base were visible via the traditional or Type I LP. Type II, III, and IV LP provided exposure to increasingly remote sites of the pharynx and supraglottic larynx. The additional exposure provided by each type of LP was consistent across all five cadaver specimens.
Conclusion
Our system catalogs the additional exposure of both cephalic and caudal tumor sites associated with division of adjacent structures. This anatomic study illustrates and systematizes the structures requiring division to provide access to a given tumor location.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Originally described in 1878 by Cheever, the lateral pharyngotomy approach has been used to access benign and malignant tumors of the tonsil, base of tongue, epiglottis, oropharyngeal wall, soft palate, and supraglottic larynx. Historically, small tumors (T1 or T2) of the base of tongue and posterior pharyngeal wall tumors have been considered the most accessible from this approach [1, 2, 6].
Visualization of the entire tumor may be difficult using the traditional window for pharyngotomy between the hypoglossal nerve superiorly and the superior laryngeal nerve inferiorly. Subsequently, maneuvers to provide wider exposure have been described, including transecting the superior laryngeal nerve; removing the lateral third of the hyoid bone or the superior cornu of the thyroid cartilage; transecting the thyrohyoid ligament; dividing the digastric, stylohyoid, hyoglossus, or mylohyoid muscles; ligating the external carotid artery; or performing lateral mandibular osteotomy [6]. However, there is no systematic classification of these maneuvers and some, such as sacrifice of the superior laryngeal nerve, produce negative functional outcomes. Therefore, we describe a new classification system of lateral pharyngotomies based upon the division of additional structures to provide better access and visualization of sites within the upper aerodigestive tract.
Materials and methods
This study qualified for exempt status by the Institutional Review Board under Federal Regulations 45CFR46.102(f) definition of “Human Subjects.” Cadavers were prepared with a solution of 75.68% isopropanol, 18.92% dipropylene glycol, and 5.4% formalin. None of the cadavers had a history of benign or malignant lesions of the oropharynx, larynx, or hypopharyx. None had a history of neck surgery. No cutaneous scars of the neck were noted. Lateral pharyngotomy was performed as described by Ferris and Meyers [4] and designated as Type I LP. Three other maneuvers were then systematically performed to provide greater access. These were labeled Type II, III, and IV LP and are described below. A zero degree endoscope was placed into the pharynx to visualize the pharyngotomy incisions transorally as they were performed transcervically.
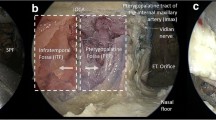
Type I pharyngotomy: a low horizontal incision along a skin crease of the neck was performed. The subcutaneous tissues and the platysma muscle were also divided. Subplatysmal flaps were raised superiorly until the submandibular gland was identified and inferiorly to the level of the clavicle. The facial vein was visualized, dissected, and divided. Blunt dissection was performed along the anterior border of the sternocleidomastoid muscle to separate this muscle from the strap muscles. The carotid sheath and its contents were visualized. Branches of the external carotid artery including the superior thyroid, lingual, and facial arteries were identified. Superiorly, the hypoglossal nerve was seen coursing anteriorly between the internal carotid artery and internal jugular vein. It was skeletonized along its length, which allowed cephalic retraction of the nerve. The contents of the carotid sheath were retracted laterally. The inferior constrictor muscle, superior pole of the thyroid gland, and superior thyroid vascular pedicle were visualized. A retractor was then placed around the posterior edge of the thyroid ala, which was rotated to the contralateral side. Blunt dissection was used to identify the origin of the superior thyroid and superior laryngeal artery in its inferomedial course and was exposed by retracting the external carotid artery. The superior laryngeal nerve was followed, after passing deep to the superior laryngeal artery it arborized into internal and external branches. The window for pharyngotomy was bordered superiorly by the hypoglossal nerve and inferiorly by the superior laryngeal nerve. The hyoid bone generally coursed in the midline of the window. The inferior constrictor muscle was then transected over the posterior edge of the thyroid ala. The pharyngeal mucosa was opened. The ability to visualize certain structures (epiglottis, ipsilateral and contralateral base of tongue, postcricoid area, arytenoids, uvula, soft palate, and vallecula) through the pharyngotomy was recorded (Figs. 1a, 2a).
Cadaver dissection progressively illustrates the classification of lateral pharyngotomy: Type I (a), Type II (b), Type III (c), and Type IV (d). XII, hypoglossal nerve, LA lingual artery, sln superior laryngeal nerve, SLA superior laryngeal artery, STA superior thyroid artery. Medical illustrations courtesy of Douglas Denys, MD, FACS
Type II pharyngotomy: Type I pharyngotomy was performed as described above. Division of the superior laryngeal artery was performed. The length of additional exposure inferior to the superior laryngeal nerve to the upper border of the thyroid ala was noted and visualized anatomic landmarks were recorded (Figs. 1b, 2b).
Type III pharyngotomy: Type I and II pharyngotomies were performed. In addition, the digastric and stylohyoid muscles were divided. The length of additional exposure through the pharyngotomy as well as the visualized anatomic landmarks was noted (Figs. 1c, 2c).
Type IV pharyngotomy: Type I, II, and III pharyngotomies were performed. In addition, the mylohyoid and hyoglossus muscles were divided. The length of additional exposure through the pharyngotomy and visualized anatomic landmarks were noted (Figs. 1d, 2d).
Results
After performing Type I LP, the epiglottis and ipsilateral base of tongue were visualized in all specimens. We performed three modifications of the traditional lateral pharyngotomy approach (Type II, III and IV) as discussed above. With the addition of Type II LP, the postcricoid area and arytenoids were also visible. Type III LP allowed visualization of all the base of tongue, the uvula, and the soft palate. Type IV LP provided additional visualization of the vallecula. For base of tongue exposure specifically, Type I and Type II LP exposed the ipsilateral base of tongue, while Type III and IV LP exposed the entire base of tongue (see Table 1 for summary). Visualization of the mucosal incisions using a transoral zero degree endoscope was possible in all cadavers.
Discussion
Within the lateral pharyngotomy approach, several alterations have been described [8, 9]. Laccourreye described the “extended lateral pharyngotomy” for resection of lesions of the lateral tongue base after induction chemotherapy. Four steps were described: (1) removal of the lateral wing of the hyoid bone, (2) transection of the digastric muscle, stylohoid muscle, and ansa hypoglossi including its branches to the mylohyoid and infrahyoid muscles, (3) ligation of the first two branches of the lingual artery, and (4) transection of the lateral floor of the oral cavity [7]. Laccourreye was able to expose superior structures including the entire mobile tongue and the soft palate, but did not mention exposure of inferior structures, such as the postcricoid area and the arytenoids. These structures were visible in Type II, III, and IV LP.
Several approaches including suprahyoid, transhyoid, and subhyoid pharyngotomy; lateral pharyngotomy; mandibulotomy; transoral laser microsurgery have been described to approach tumors of the oropharynx and supraglottis. Postoperative swallowing without aspiration, speech quality, length of stay, complications, costs and oncologic safety of these methods have been compared [2, 5, 11, 12]. Our modifications differ from previously described approaches. We chose to include ligation of the superior laryngeal artery instead of branches of the lingual artery. Sacrifice of the superior laryngeal nerve is rarely needed [1], and results in significant functional deficits. We did not need to sacrifice the superior laryngeal nerve in any of the cadavers to facilitate exposure. We did not include removal of the lateral hyoid bone, but did transect the digastric and stylohyoid muscles. We were able to expose similar structures as Laccourreye without disrupting the hyoid bone.
The advent of transoral robotic surgery (TORS) has allowed for resection of many of these tumors with the aim to reduce potential quality of life deficits from previous surgical modalities but with equal or better oncological outcomes [5, 11, 12]. Lateral pharyngotomy can be used as an adjunct to TORS if suboptimal transoral access occurs; this modality can also be utilized as an alternative to TORS when tumor visualization is poor pre-operatively [10]. Furthermore, the extended lateral pharyngotomy was shown useful for lateral tongue base tumors [7]. Tumors of this region are not amenable to transoral endoscopic excision and many surgical centers are not trained in this skill; therefore, LP may be useful in this situation. Additionally, the transhyoid approach is useful for approaching tumors of the base of tongue, but not tumors that extend from the base of tongue to the vallecula [1]. LP would be more appropriate in these patients. In our study, Type IV pharyngotomy provided exposure of the vallecula.
Tumors of the oropharynx and supraglottis vary widely and no one (exclusively transoral or transcervical) approach is appropriate for all. Experienced surgeons utilize a variety of approaches tailored to the patient and the tumor. Adapting an emphasis on functional outcomes produced by a transoral approach to a systematic open LP approach allows more tools to control the tumor while preserving function. With transoral approaches, simultaneous neck dissection is often necessary [4]. For those patients requiring neck dissetion, exposure of vascular structures for pre-TORS ligation is facilitated. Additionally, LP as described in this study, is readily accomplished, expanding visualization and margin control which can be limited in an exclusively transoral approach. As in staging of head and neck malignancies, a widely accepted classification system facilitates comparison of incidence, treatment, and outcomes. We were interested in creating a classification system of LP to assist with documentation and communication. This system can simplify the choice of extension of the traditional approach depending on the structure in need of visualization.
Conclusion
A variety of modifications of the LP approach may be used for surgical excision of upper aerodigestive tract lesions. A classification system can provide consistency in surgical documentation, comparison of end results, and communication regarding patient care. Critically, knowledge of the structures at risk during the surgical dissection allows a more accurate risk–benefit discussion with the patient. Our system catalogs the additional exposure of both cephalic and caudal tumor sites associated with division of adjacent structures. This will allow a standardized understanding of structures to be sacrificed to facilitate adequate surgical exposure to the targeted areas.
References
Carrou R, Soose R (2008) Lateral pharyngotomy. Myers operative otolaryngology—Head and Neck Surgery 2008 Saunders, Philadelphia
Cheever D (1878) Cancer of the tonsil: removal of the tumor by external incision (A second case). Boston Med Surg J 99:133–139
Chung T, Rosenthal E, Magnuson J, Carroll W (2014) Transoral robotic surgery for oropharyngeal and tongue cancer in the United States. Laryngoscope. 2014; online
Ferris R, Myers E (2005) Suprahyoid pharyngotomy. Op Tech Otolaryngol 16: 49–54.
Ford S, Brandwein-Gensler M, Carroll W et al (2014) Transoral robotic versus open surgical approaches to oropharyngeal squamous cell carcinoma by human papillomavirus status. JAMA Otolaryngol Head Neck Surg 139(12):1290
Holsinger F, Laccourreye O, Weber R (2005) Surgical approaches for cancer of the oropharynx. Op Tech Otolaryngol 16: 40–48
Laccourreye O, Seccia V, Ménard M et al (2009) Extended lateral pharyngotomy for selected squamous cell carcinomas of the lateral tongue base. Ann Otol Rhinol Laryngol 118(6): 428–434
Orton H (1930) Lateral transthyroid pharyngotomy: Trotter’s operation for malignant conditions of the laryngopharynx. Arch Otolaryng 52: 321–338
Stem S (1992) Anatomy of the lateral pharyngotomy approach. Head Neck 14:153–156
Tompkins J, Mohamed F, Wood C et al (2014) Lateral pharyngotomy for management of base of tongue cancer. In: Presented at IFHNOS 5th World Congress/AHNS 2014 (poster), New York, NY, July 26–30, 2014
Weinstein G, O’Malley B Jr, Magnuson J et al (2012) Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope 122(8):1701–1707
White H, Ford S, Bush B et al (2013) Salvage surgery for recurrent cancers of the oropharynx: comparing TORS with standard open surgical approaches. JAMA Otolaryngol Head Neck Surg 139(8):773–778
Acknowledgements
Thank you to those that donated their bodies and to their family members for allowing this research to be possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial support
This research received no internal or external grant funding.
Conflict of interest
The authors report no relevant financial disclosures related to this current work.
Rights and permissions
About this article
Cite this article
Shires, C., Smith, A., Lee, J. et al. Classification system for lateral pharyngotomy: systematic study of anatomic exposure in a human cadaver model. Surg Radiol Anat 39, 975–979 (2017). https://doi.org/10.1007/s00276-017-1827-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-017-1827-x