Abstract
Background
Only a few studies, involving small numbers of patients, have globally assessed the curvature of the thoracic aorta but without any details concerning the location of the supra-aortic trunks.
Objectives
Using CT to describe normal aortic-arch morphology and its changes with age and sex.
Methods
344 CT scans were studied. We measured the distances from the apex to the ascending and descending aorta, the curvilinear length of the entire arch, that of the segment, including bifurcations of supra-aortic vessels, and the angle, height, and shift of the arch.
Results
In men, the arch was significantly longer (146.2 vs 122.8 mm; p < 0.001), higher (49.3 vs 40.1 mm, p < 0.001), and wider transversely (83.6 vs 73.3 mm; p < 0.001) than in women. The average men’s arch also had a more acute angle at the apex (79.7° vs 83.7° p < 0.001). Neither morphology nor age influenced the winding angle around the mediastinum. Aging was accompanied by deflection and extension of the aortic arch, which grew more anteroposteriorly (6.1 mm/10 years in men) than vertically (2.5 mm/10 years in men), while the apex moved towards the rear of the arch. The ascending aorta was the only curvilinear length unaffected by age, whereas the supra-aortic trunks parted from each other.
Conclusion
We believe that all these original observations could lead to a better assessment of normal aging of the aorta and guide technical choices during surgical or hybrid procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aorta’s functions vary from an absorbing structure of the pulse wave to a conducting vessel. The intrathoracic segment, while reducing systolic impact, accomplishes a half-turn within a reduced space and giving rise to the supra-aortic trunks. Various diseases and age are responsible for histological modifications to the structure of the aortic wall [14, 16–18] and several studies have observed increased thoracic-aorta diameters at various levels [4, 7, 11, 19]. However, there is still scant knowledge of the morphology of aortic arches and of segment lengths where the supra-aortic trunks arise. Only a few studies, involving small numbers of patients, have assessed the curvature of the thoracic aorta [3, 8, 13]. The most recent published study [1] is comprehensive with regard to the three-dimensional aortic-arch morphology but does not specify the location of the supra-aortic trunks. The authors aimed to characterize a population without any direct influence of aortic root disease. To fully assess the anatomy of the thoracic aorta and, more specifically, the segments of the supra-aortic trunks, we investigated the changes in the length, diameter, and curvature of the thoracic aorta in a large patient sample based on a general population.
Materials and methods
Patients
All computer tomography scan (CT scan) examinations requested by a non-cardiovascular department between 1 January 2012 and 1 June 2013 in our University Hospital were examined. The exclusion criteria were any history of heart or thoracic surgery, aortic syndrome, aneurysm, a disease of the great vessels, and Marfan syndrome. Given the high prevalence of hypertension, we did not exclude patients suffering from these diseases; moreover, in a previous study, Lin [7] did not found any statistical link between systolic blood pressure and aortic root or ascending aorta diameters. Likewise, as we aimed to study a general population (instead of a normal one), patients with uncomplicated atheroma or diabetes were not excluded, owing to the high prevalence of this type of pathology. Patients were retrospectively identified from our hospital’s Radiology Information System (Xplore, EDL, La Seyne-sur-Mer, France). They were divided into two groups according to sex. For each patient, age at the CT scan examination date was recorded and, when available, body mass and height data. Body-mass index (BMI) and body-surface area (BSA) [10] were calculated from these variables. A blind analysis of the CT scan examination was performed from demographic and age data. This descriptive, retrospective, observational monocentric study was conducted according to ethical principles for medical research involving human subjects in French university hospitals [2].
Computer tomography scan examination and analysis
The examinations were conducted with a CT scan General Electric Healthcare Discovery HD750. Constants for X-ray Tube were tube voltage: 100 kV (120 for patients with a high B.M.I.) and a charge automatically adapted, gantry rotation time vary from 0.4 to 0.7 s, average collimation was 0.625, field of view varies from 350 to 550 mm, and slice thickness was between 0.625 and 2 mm. Injection of 90 mL of Xenetix 350® (Guerbet® France) was performed at 2.5 mL per second, on the arm previously equipped with intravenous access. It has not been required that it be placed on the right arm. Scans were analysed on a General Electric Advantage Workstation 4.4 (General Electric, Waukesha, Wisconsin, USA), using a semi-automatic segmentation of the selected thoracic-aorta segment. Measurements were taken by a single operator.
Measurements
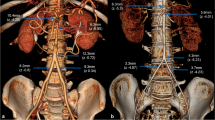
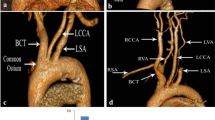
Aortic segmentation was performed through a curvilinear reconstruction of the aortic arch, determining the centre of the blood flow in the lumen automatically. A manual re-contouring of the lumen addressed errors in segmentation. We used multiplanar rendering in double oblique, curvilinear reformatting, and axial view, perpendicular to the curvilinear reformatting. Like many other studies, we took the pulmonary artery as anatomical landmark [1, 19]. As the study on a large cohort of Rogers et al [11], we focused on a part located between (A) the lumen centre point in ascending aorta at the mid-level of the right pulmonary artery flow [11] and the corresponding point of the descending aorta (D) (Figs. 1, 2). These two points were our main anatomical landmarks, combined with:
-
the most cranial point (C) in the aortic arch: the apex of the arch;
-
the point corresponding to the anterior edge of the brachiocephalic arterial trunk (BCT);
-
the point corresponding to the posterior edge of the left common carotid artery (LCCA);
-
the point corresponding to the posterior edge of the left subclavian artery (LSCA).
We also determined the following extravascular points. Cp was the point corresponding to the orthogonal projection of point C on the transverse length of the aortic arch (AD), so that the rectilinear transverse length of the aortic arch AD and the aortic-arch height CCp were perpendicular (Fig. 2). Point Cs is the orthogonal projection of C onto the axial plane passing through A to D.
Using the coordinates of A, C, D, Cp, and Cs, the following measurements were calculated:
-
rectilinear transverse length AD, rectilinear ascending length AC and rectilinear descending length CD, arch height CCp, and the shift (ACp/CpD ratio);
-
the angle of the aortic arch in the axial plane ACsD (helix angle);
-
the apex angle ACD.
We recorded the mean aortic diameters at points A and D obtained from the circulating surface, in a true short-axis plane, orthogonal to blood flow centreline. We also collected data on the various curvilinear lengths at mid-flow that characterized the aortic arch.
-
The length of the entire studied arch from point A to point D (Lg arch) and the length of the segment of the supra-aortic trunks (Lg BCT–LSCA).
-
The length between point A and the brachiocephalic arterial trunk (Lg A–BCT).
-
The length between the LSCA and point C (Lg LSCA–C); this distance was expressed negatively if point C was proximal to the posterior plan of the left subclavian artery implantation and was recorded as positive if it was positioned distally.
-
The length between point C and point D in the descending aorta (Lg C–D).
Because our study allowed us to estimate morphological aging of the aortic arch, we also conducted linear regression analyses on the speed at which the variables changed over time.
Comparison with subjects with aortic dissection
The biometric measurements of the normal aortic arches were used as a reference for a comparison with afterward obtained values (“CT patterns of acute type A aortic-arch dissection: longer, higher, and wider” actually submitted) using the same reference points on dissected aortas. These parameters were measured in 57 patients (45 men) who had a contrast-enhanced thoracic CT scan requested by the heart surgery team for aortic type A dissection.
Statistical analysis
All measurements described above were recorded for all patients. The data were then stratified according to age or a continuous feature that separated them into classes, such as age or height. The categorical variables were described by their size and proportions. Continuous data are presented by the mean and 95% confidence interval, unless stated otherwise. The distribution of variables was evaluated systematically by a test of normality. The alpha risk was set at 5% and was not corrected for multiple testing.
The comparison of means between the different measurement points was performed by the analysis of variation (ANOVA) of matched samples. Qualitative variables were compared using a Fisher’s exact test. Comparisons between groups were initially performed without adjustment, using a Student’s t test or Wilcoxon test for continuous variables with a normal or other distribution, respectively. Another analysis was performed after adjusting for age, sex, and body weight and height using a general linear model. Finally, we separated age quartiles for both sexes and compared average arches of young patients with average arches of older ones and presented the graphically the results of the comparison.
To ensure the measurements were valid, the single operator in charge of the data collection trained on ten CT scans. These results were excluded from the analyses. Thereafter, the stability of the aortic-arch measurements was evaluated by repeating the measurements of 34 randomly selected subjects three times. Two measurements on the same patient were made after a minimum interval of 10 days. The obtained measurements were compared within each pair of successive iterations (1 and 2, then 2 and 3). Only measurements from iteration 3 were retained. For each measured variable, the disparity between two successive measurements was tested by Student’s t test over the matched series; quantization matching was done using the Shrout–Fleiss intraclass correlation coefficient [15]. All statistical analyses were performed with the SAS software (SAS 9.3, SAS Institute Inc., Cary, NC, USA). All were double-tailed tests (except for ANOVA), and the alpha risk was set at 0.05.
Results
The contrast-enhanced thoracic CT scans of 344 patients (162 female, 182 male) were analysed. Data for body mass were available for 243 patients and height for 202. All demographic variables and those relating to arch morphology were normally distributed. The demographic variables were divided as shown in Table 1. There were statistically significant differences in body mass, height, and BSA between the two groups. However, BMI and age range did not significantly differ between sexes.
Reproducibility of measurements
We assessed the reproducibility of the aortic measurements in a sample of 34 patients. The first series of measurements (iteration 1) was statistically different from iteration 2 for several variables. The only discrepancy between iterations 2 and 3 was the diameter at the level of the BCT. The average difference between measurements 2 and 3 was 0.56 mm ± 0.67. Between these two iterations, the intraclass correlation coefficient was always >0.7 for all morphologic variables.
Aortic-arch morphology
Univariate analyses of the morphologic differences in the aortic arch according to sex revealed significant differences for most of the measurements (Table 2). The men had a statistically longer (Lg arch), higher (CCp), and transversally more extended (AD) arch than did the women. In addition, the men’s average aortic arch had a sharper angle at the apex.
Demographic data compared to the morphology of the aortic arch
The correlations between demographic data and measurements of the aortic arch were studied for each group. The morphologic variables that were significantly correlated with the same demographic variable, irrespective of sex, were as follows. The distance between the subclavian artery and the apex was statistically associated with body mass: heavier patients had a subclavian artery placed further behind the apex of the arch. The curvilinear length from point A to BCT was only positively correlated with body mass (women p = 0.041; men p < 0.001). The angle at the top of the arch was correlated with body mass in both sexes (women p = 0.034, men p = 0.002), with a more open angle in heavier subjects. The shift was independent of the demographic data, as was the helix angle of the aorta around the mediastinum.
Effect of age on aortic-arch morphology
Age was significantly correlated with most of the morphological variables apart from ascending aorta curvilinear length and apex and helix angle. The shift, regardless of demographic data, was statistically associated with age irrespective of sex. The modelled velocities obtained from linear regression of the morphological variables according to age are shown in Table 3. These modelled velocities differed according to the portion of the arch assessed and to sex. The segment of the aorta where the supra-aortic trunks arose had weaker growth compared to the rest of the aortic arch. The height of the arch also increased more slowly than the anteroposterior length.
According to methods, the age quartiles for both sexes were separated out and the data from average arches of young patients were compared with those for average arches of older patients (Fig. 3). We choose point A as a reference for comparison. For both sexes, graphical analysis showed a non-super-imposable evolution of the arch during aging.
Discussion
General considerations
The involvement of sex and age in the morphology of the thoracic aorta has widely been documented [7, 11, 12, 19]. In contrast, neither smoking nor hypercholesterolemia has any demonstrated effect on aortic morphology [19]. Given the high prevalence of these characteristics, we focused our work on a general patient sample. The selected markers were those already widely used elsewhere [1, 11, 19]. The most recent publication on this topic [1] was based on the midpoint of the segment (AD), but we elected to use the apex of the aortic arch to define the curvature of the aortic arch.
Aortic-arch morphology according to gender
We observed an increase in the diameters and curvilinear and straight lengths of the arch with aging. However, these changes were not identical, since non-homothetic distortion of the aorta was observed. In agreement with other reports, we found that sex affected aortic diameter [5, 7, 11, 19]. In a study of a cohort of 70 patients, Hager et al. [5] reported various aortic diameters in various positions and correlations between these diameters and the patients’ age. Their results were very similar to ours. The larger study of Rogers et al. [11] also reported diameters similar to ours, despite different points of reference. In a smaller sample, Rylski et al [12] reported diameters similar to ours on the ascending, horizontal, and descending aorta. However, they provided no detailed data on horizontal aorta.
Understanding of the overall anatomy of the aortic arch was greatly advanced by the recent article of Cascario et al [1], which, from identical landmarks in the ascending and descending aorta, showed numerous patterns of aortic morphology. Total lengths and diameters were consistent with our measurements. The authors also observed a shift that was calculated by a different method from ours, but finally, they found an asymmetric aortic morphology.
Aortic-arch morphology according to age
Although the design of our study was not the most suitable to analyse changes in aortic morphology during aging, it did allow us to estimate them from the variability in age of our patients. In men and women, comparison of arches in the youngest age quartile and the oldest quartile showed a number of variations. The aortic arch extended more in the anteroposterior direction than vertically. In addition, the ascending rectilinear length increased faster than the descending rectilinear length; there was a shift towards the front of the horizontal portion of the arch, whereas the apical point was located further behind the arch. Moreover, the angle at the top of the arch increased. Consequently, the aortic arch underwent curve deflexion with age. These findings are in line with those of Craiem et al. [3]. No modification was observed with age regarding the distance between reference point A and the anterior plane of the insertion of the brachiocephalic arterial trunk (curvilinear length of the ascending aorta). This length was not correlated with any of the demographic variables, apart from BSA. The lack of association with other demographic variables could be due to a lack of statistical power, especially among the female group. The increased length of the emerging segment of the supra-aortic trunks was not the same as that in the entire aortic arch. Aortic-arch growth was faster within the segments without any collateral arteries than in the segments with the supra-aortic trunks. These observations are similar to those of Craiem and Rylski [3, 12]. Craiem demonstrated that aging leads to unfolding of the thoracic aorta with an increased width and a reduced tortuosity. Rylski used three reference points different from ours. Consequently, we cannot compare our results to theirs. In their study, Craiem underlines the possible effect of aortic arch unfolding on aortic-arch physiopathology. We observed similar changes in diameter over time to those of Hager et al. [5]. The difference in the rate of diameter growth was greater for the furthermost points than for the supra-aortic trunk, which is a similar result to that of Hager et al. The rates found were also similar to those previously reported by Rylski [12].
Frydrychowicz et al [5] worked on 4D MRI flow in the aortic arch, studying age, and aortic morphology. They were able to link the aortic flow pattern geometry with both the age of patients and aortic morphology. This lends weight to our findings which suggest an evolution of aortic-arch morphology with age. However, we cannot answer the question of whether morphologic preceded haemodynamic flow pattern modifications or vice versa. We hypothesize that the change in aortic morphology results from the intermingling of several factors of which age and flows in the aortic arch are only elements.
Positioning of the supra-aortic trunks
The apical point of the arch (C) is the crucial element of the aortic arch. Yet, in young patients, we found that it was positioned proximally to the left subclavian artery. Unlike the supra-aortic trunks, which followed a different pattern, it shifted backwards towards the aortic arch with aging. Among older patients, therefore, it is placed distally to the left subclavian artery. It seems that the aortic arch, while undergoing a general deflection, extends within the portion situated distally to the supra-aortic trunks. The portion situated proximally to these trunks does not extend, whereas the segment where the supra-aortic trunks arise increases more slowly than the descending portion. Initially positioned on both sides of the apex, the three supra-aortic trunks were situated proximally to point C among the oldest patients.
Limitations
Several publications have demonstrated the superiority of a semi-automatic assessment of the flow centre, as the more reproducible methods of measuring flow length [11] (comparison methods included the assessment by an expert reviewer). Our measuring method appeared robust, and the intraclass correlation coefficients were all >0.7, with most being >0.9. This high concordance produced excellent reproducibility during repeated measurements [15]. The definition of a normal arch is not universally acknowledged, and hence, inclusion or exclusion criteria can vary between studies. Having the measurements performed by a sole operator may have influenced our results. Furthermore, the reference points A and D may have been related to parameters that were not considered in this work, such as shifting of the pulmonary artery with age, and overall modification of thoracic morphology. Our results do not allow us to consider variations in the entire thoracic aorta from the valvular plane to the aortic hiatus. However, concerning the portion with the supra-aortic trunks, we obtained results that we regard as reliable. In addition, when our findings are compared with those from other studies that used different points of reference, the results are similar, which tends to minimize our limitations.
Clinical applications
The findings presented are a preliminary to a larger study comparing the morphology of normal and dissected aortic arches. The use of thoracic stents in daily practice has generally been restricted to the segment of the descending thoracic aorta. One of the limitations of endovascular stenting on the aortic arch is its particular shape, which may lead to a lack of apposition of the stent along the aortic wall. This may lead to an endoleak (for stent-grafts), and to a collapse of the stent [6, 9]. The results of our work will allow the development of medical devices specifically designed for the aortic arch.
Conclusion
Many parameters, not previously investigated, are described in this study of the aortic arch. We observed a significant change in the morphology of the aortic arch with aging. Diameters increased, the arch curvature lessened, and the bases of the supra-aortic trunks moved towards the front of the arch apex. These results could be used to evaluate a pathological aorta (suffering from aneurysm or dissection) and to design new implantable devices.
Abbreviations
- AC:
-
Ascending aortic-arch length
- ACD:
-
Apex angle of the aortic arch
- ACsD:
-
Helix angle of the aortic arch
- AD:
-
Transverse length of the aortic arch
- BCT:
-
Brachiocephalic arterial trunk
- BMI:
-
Body mass index
- BSA:
-
Body-surface area
- CCp:
-
Aortic-arch height
- CD:
-
Descending length of the aortic arch
- CDA:
-
Descending angle of the aortic arch
- CT Scan:
-
Computed tomography scan
- DAC:
-
Ascending angle of the aortic arch
- LCCA:
-
Left common carotid artery
- LSCA:
-
Left subclavian artery
- PACS:
-
Picture archiving and communication system
References
Casciaro ME, Craiem D, Chironi G et al (2014) Identifying the principal modes of variation in human thoracic aorta morphology. J Thorac Imaging 29:224–232. doi:10.1097/RTI.0000000000000060
Claudot F, Alla F, Fresson J et al (2009) Ethics and observational studies in medical research: various rules in a common framework. Int J Epidemiol 38:1104–1108. doi:10.1093/ije/dyp164
Craiem D, Chironi G, Redheuil A et al (2012) Aging impact on thoracic aorta 3D morphometry in intermediate-risk subjects: looking beyond coronary arteries with non-contrast cardiac CT. Ann Biomed Eng 40:1028–1038. doi:10.1007/s10439-011-0487-y
Garcier J-M, Petitcolin V, Filaire M et al (2003) Normal diameter of the thoracic aorta in adults: a magnetic resonance imaging study. Surg Radiol Anat 25:322–329. doi:10.1007/s00276-003-0140-z
Hager A, Kaemmerer H, Rapp-Bernhardt U et al (2002) Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg 123:1060–1066. doi:10.1067/mtc.2002.122310
Hinchliffe RJ, Krasznai A, Schultzekool L et al (2007) Observations on the failure of stent-grafts in the aortic arch. Eur J Vasc Endovasc Surg 34:451–456
Lin FY, Devereux RB, Roman MJ et al (2008) Assessment of the thoracic aorta by multidetector computed tomography: age- and sex-specific reference values in adults without evident cardiovascular disease. J Cardiovasc Comput Tomogr 2:298–308. doi:10.1016/j.jcct.2008.08.002
Malkawi AH, Hinchliffe RJ, Yates M et al (2010) Morphology of aortic arch pathology: implications for endovascular repair. J Endovasc Ther 17:474–479
Melissano G, Bertoglio L, Civilini E et al (2007) Results of thoracic endovascular grafting in different aortic segments. J Endovasc Ther Off J Int Soc Endovasc Spec 14:150–157. doi:10.1583/1545-1550(2007)14[150:ROTEGI]2.0.CO;2
Mosteller RD (1987) Simplified calculation of body-surface area. N Engl J Med 317:1098. doi:10.1056/NEJM198710223171717
Rogers IS, Massaro JM, Truong QA et al (2013) Distribution, determinants, and normal reference values of thoracic and abdominal aortic diameters by computed tomography (from the Framingham Heart Study). Am J Cardiol. doi:10.1016/j.amjcard.2013.01.306
Rylski B, Desjardins B, Moser W et al (2014) Gender-related changes in aortic geometry throughout life. Eur J Cardiothorac Surg. doi:10.1093/ejcts/ezt597
Rylski B, Blanke P, Beyersdorf F et al (2014) How does the ascending aorta geometry change when it dissects? J Am Coll Cardiol. doi:10.1016/j.jacc.2013.12.028
Schlatmann TJ, Becker AE (1977) Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol 39:13–20
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428
Stefanadis C, Stratos C, Vlachopoulos C et al (1995) Pressure-diameter relation of the human aorta. A new method of determination by the application of a special ultrasonic dimension catheter. Circulation 92:2210–2219
Stefanadis C, Dernellis J, Vlachopoulos C et al (1997) Aortic function in arterial hypertension determined by pressure-diameter relation: effects of diltiazem. Circulation 96:1853–1858
Stefanadis C, Dernellis J, Tsiamis E et al (2000) Aortic stiffness as a risk factor for recurrent acute coronary events in patients with ischaemic heart disease. Eur Heart J 21:390–396. doi:10.1053/euhj.1999.1756
Wolak A, Gransar H, Thomson LEJ et al (2008) Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 1:200–209. doi:10.1016/j.jcmg.2007.11.005
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. This descriptive, retrospective monocentric study was conducted according to ethical principles for medical research involving human subjects in French university hospitals (Claudot F, et al. Ethics and observational studies in medical research: various rules in a common framework. Int J Epidemiol 2009, 38(4):1104–1108.)
Rights and permissions
About this article
Cite this article
d’ostrevy, N., Ardellier, F.D., Cassagnes, L. et al. The apex of the aortic arch backshifts with aging. Surg Radiol Anat 39, 703–710 (2017). https://doi.org/10.1007/s00276-016-1792-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-016-1792-9