Abstract
Purpose
The sulci constituting the structure of the pars triangularis and opercularis, considered as ‘Broca’s area’, present wide anatomical and morphological variations between different hemispheres. The boundaries are described differently from one another in various studies. The aim of this study was to explore the topographical anatomy, confirm the morphological asymmetry and highlight anatomical variations in Broca’s area.
Methods
This study was performed with 100 hemispheres to investigate the presence, continuity, patterns and connections of the sulcal structures that constitute the morphological asymmetry of Broca’s area.
Results
Considerable individual anatomical and morphological variations between the inferior frontal gyrus and related sulcal structures were detected. Rare bilateralism findings supported the morphological asymmetry. The inferior frontal sulcus was identified as a single segment in 54 % of the right and two separate segments in 52 % of the left hemispheres, which was the most common pattern. The diagonal sulcus was present in 48 % of the right and 54 % of the left hemispheres. It was most frequently connected to the ascending ramus on both sides. A ‘V’ shape was observed in 42.5 % of the right hemispheres and a ‘Y’ shape in 38.3 % of the left hemispheres, which was the most common shape of the pars triangularis. Moreover, the full results are specified in detail.
Conclusions
Knowledge of the anatomical variations in this region is indispensable for understanding the functional structure and performing safe surgery. However, most previously published studies have aimed to determine the anatomical asymmetry of the motor speech area without illuminating the topographical anatomy encountered during surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pars opercularis (POP) (Broadmann’s area 44) and pars triangularis (PTR) (Broadmann’s area 45) are classically considered as Broca’s area since Paul Broca first introduced his two cases who were unable to speak after lesions enclosing the left third convolution of the frontal lobe in nineteenth century [10, 11]. In recent years, Brodmann’s areas 6 [4, 9, 24, 31] especially the ventral part [38], 46 and 47 [4, 9, 24, 31] are also considered to be involved and ‘Broca’s complex’ term has been raised. It has also been widely accepted that lesions restricted to classical Broca’s area are associated with mild, non-fluent speech deficits, whereas classical Broca’s aphasia requires involvement of the insula, periventricular white matter and lower motor cortex [2, 7]. Dominant striatum has been shown to be important in speech function, as well. Electrical stimulation of the anterior putamen causes anarthria/dysarthria, while the head of the caudate stimulation causes perseveration [23]. Furthermore, a case who did not face any speech deficits after a tumor resection involving the left inferior and middle frontal gyri extending to internal capsule and anterior insula has been reported [36].
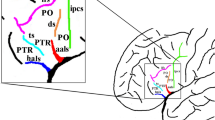
The inferior frontal gyrus (IFG) consists of orbital, triangular and opercular parts in the anteroposterior direction. The POP is separated from the PG by the inferior precentral sulcus (IPS), from the middle frontal gyrus by the IFS and from the PTR by the anterior ascending ramus (AR) of SF. The PTR is separated from the POP by the AR, from the middle frontal gyrus by IFS, and from the pars orbitalis (POR) by the anterior horizontal ramus (HR) of the SF. Occasionally, a sulcus between the AR and IPS exists in POR that can be easily distinguished from the AR and termed the ‘diagonal sulcus’ (DS) [39]. Likewise, the PTR includes ‘triangular sulcus’ (TS). These sulci were defined to separate PTR and POP from the neighboring cortical structures [14, 26, 27] and used as the anatomical boundaries of these regions [26, 27, 39, 40] in this study (Fig. 1).
Major sulci defining the borders of the pars opercularis, triangularis and orbitalis. a aSCS: anterior subcentral sulcus (blue line), AR ascending ramus of the sylvian fissure (purple line), CS central sulcus (red line), DS diagonal sulcus (pink line), FMS frontomarginal sulcus (brown line), FOS frontoorbital sulcus (orange line), HR horizontal ramus of the sylvian fissure (purple line), IFS inferior frontal sulcus (black line), IPS inferior precentral sulcus (white line), SF sylvian fissure (purple line), TS triangular sulcus (green line). b POP: pars opercularis (the area within the confines of the yellow line), POR pars orbitalis (the area within the confines of the blue line), PTR pars triangularis (the area within the confines of the pink line) (color figure online)
Because Broca’s area is an essential cortical region for expressive speech, its protection during surgery is mandatory. The aim of this study was to investigate the detailed sulcal anatomy of Broca’s area and neighboring structures in light of previously described anatomical definitions, compare the left and right hemispheres in terms of morphological asymmetry and examine the inconsistency between nomenclatures.
Methods
This study was performed using 100 cerebral hemispheres from 50 adult cadavers submitted to autopsy at the Neurosurgery Department, Uludağ University School of Medicine, between January 2008 and February 2013. Twenty-three of the cadavers were women, and 27 of the cadavers were men; all cadavers were between the ages of 20–77 years. The autopsies were performed between the 2nd–6th hours postmortem. The specimens were fixed in 50 % formalin solution.
Dissections were performed at the Microneurosurgery Laboratory of Neurosurgery Department of Uludağ University School of Medicine after authorization by the institution’s Ethics Committee. All specimens were fixed in formalin for at least 2 months before dissection. The dura, arachnoid membranes and superficial vessels of the hemispheres were removed with the aid of an Opmi Pentero microscope (X40), (Carl Zeiss Inc., Oberkochen, Germany) and photographed. Subsequently, the related sulcal and gyral structures were marked using the PhotoScape 3.6.5 software.
Results
Precentral sulcus
The precentral sulcus (PS) consisted of two segments in 54 % (27/50), three segments in 42 % (26/50), and four segments in 4 % (2/50) of the right hemispheres; and of two segments in 54 % (27/50), three segments in 38 % (19/50), and four segments in 8 % (4/50) of the left hemispheres (Fig. 2a, b). This sulcus consisted of two segments in 26 % (13/50), three segments in 16 % (8/50), and four segments in 2 % (1/50) of the brains, bilaterally. There were dual parallel inferior precentral sulci (IPS) in 6 % (3/50) of the right and 4 % (2/50) of the left hemispheres (Fig. 2c). One of them (% 2) was dual parallel bilaterally. The IPS was found not to reach the SF in each of the hemispheres on both sides (100 %).
The form of IPS can be determined on non-dual parallel sulci and was studied on 47 of the right, 48 of the left hemispheres. It was determined to be ‘arcuat’ in 29.8 % (14/47), ‘ramified’ in 25.5 % (12/47), ‘bayonet’ in 23.4 % (11/47), and ‘Y’ in 21.3 % (10/47) of the right hemispheres. In the left hemispheres, the results were, respectively, 29.2 % (14/48), 31.3 % (15/48), 27.1 % (13/48), and 12.5 % (6/48) (Fig. 3a–d). The ‘ramified’ form was found to occur in 6.3 % (3/47) of cases bilaterally, while each of the other three forms occured in 4.3 %.
Inferior frontal sulcus
The IFS was identified as a single continuous segment in 54 % (27/50) of the right and 48 % (24/50) of the left hemispheres. In 46 % (23/50) of the right and 52 % (26/50) of the left hemispheres, it was observed as two separate, discontinuous segments. ‘True connection’ was demonstrated between the posterior end of the IFS and the IPS in 66 % (33/50) of the right and 58 % (29/50) of the left hemispheres, while 34 % (17/50) of the right and 42 % (21/50) of the left hemispheres showed no connection. Among specimens with true connection; 20 (6.1 %) of the right and 15 (51.7 %) of the left were ‘true long’, while 13 (39.4 %) of the right and 14 (48.3 %) of the left were ‘true short’ (Fig. 4a–c). We did not observe ‘pseudoconnection’ in our hemispheres. The IFS was connected to the frontoorbital (FOS) and the DS in 12 % (6/50), the frontomarginal sulcus (FMS) in 18 % (9/50), and the AR in 4 % (2/50) of the right hemispheres. The corresponding percentages in the left hemispheres were 6 % (3/50), 8 % (4/50), 8 % (4/50), and 10 % (5/50), respectively (Fig. 5a–d).
The connections between the inferior frontal and inferior precentral sulci. IFS inferior frontal sulcus, IPS inferior precentral sulcus. a IFS (red line) is connected to IPS (white line) via ‘true long’ connection. b IFS (red line) is connected to IPS (white line) via ‘true short’ connection. c There is no connection between the IFS (red line) and IPS (white line) (color figure online)
Inferior frontal sulcus connections with the neighboring sulci. AR ascending ramus of the sylvian fissure, DS diagonal sulcus, IFS inferior frontal sulcus, FMS frontomarginal sulcus, FOS frontoorbital sulcus. a IFS is connected with FMS. b IFS is connected with FOS. c IFS is connected with DS. d IFS is connected with AR
Anterior ascending ramus
The AR of the SF was observed in 98 % (49/50) of the left and 100 % (50/50) of the right hemispheres (Fig. 6b).
Horizontal ramus
The lack of the HR was observed in three (6 %) of the right and two (4 %) of the left hemispheres, one of which was undetected bilaterally (2 %) (Fig. 6b).
Diagonal sulcus
Diagonal sulcus was present in 48 % (24/50) of the right and 54 % (27/50) of the left hemispheres. Connections with AR were present in 26 % (13/50) of the right and 34 % (17/50) of the left hemispheres. The connection with IFS was 12 % (6/50) on the right and 8 % (4/50) on the left. We did not observe connections with IPS on either sides. The DS was connected with the AR in 10 % and the IFS in 4 % of the brains bilaterally. It was bilaterally unconnected to any neighboring sulcal structures in 10 % (5/50) of the specimens (Fig. 7a–c).
Diagonal sulcus connections with the neighboring sulci. AR ascending ramus of the sylvian fissure, CS central sulcus, DS diagonal sulcus, HR horizontal ramus of the sylvian fissure, iIFS inferior extension of the inferior frontal sulcus, IFS inferior frontal sulcus, IPS inferior precentral sulcus, TS triangular sulcus. a DS is connected with AR (red star). b DS is connected with IFS (red star). c DS is not connected to any of the neighboring sulci (color figure online)
Triangular sulcus
Triangular sulcus was observed in 66 % (33/50) of the right and 68 % (34/50) of the left hemispheres (Fig. 7a). It was observed in 42 % (21/50) and absent in 14 % (7/50) of the brains bilaterally.
Inferior extension of the inferior frontal sulcus
We observed the presence of a short branch of the inferior frontal sulcus (iIFS) in the PTR in 78 % of the right and 68 % of the left hemispheres (Fig. 7a). This small branch was observed bilaterally in 46 % (23/50) of the brains.
Pars triangularis shape
The shape of the PTR could be examined in 47 brains due to either the lack of HR and AR or the dual parallel inferior precentral sulcus in those brains. Also, bilateralism was examined in 45 brains for the same reason. ‘V’ shaped PTR was seen in 42.5 % (20/47) of the right and, 36.1 % (17/47) of the left hemispheres. ‘U’ shaped PTR was seen in 40.5 % (19/47) of the right and, 25.5 % (12/47) of the left specimens. Similarly, ‘Y’ shaped PTR was detected in 17 % (8/47) of the right and in 38.3 % (18/47) of the left hemispheres. The brains presented ‘V’ shaped PTR in 22.2 % (10/45), ‘U’ shaped in 15.5 % (7/45), and ‘Y’ shaped in 6.6 % (3/45) of the hemispheres, bilaterally (Fig. 8a–d).
Pars triangularis shape. AR ascending ramus of the sylvian fissure, HR horizontal ramus of the sylvian fissure, IPS inferior precentral sulcus. a ‘V’ shaped pars triangularis (white line). b ‘U’ shaped pars triangularis (white line). c ‘Y’ shaped pars triangularis (white line). d Shape of pars triangularis cannot be determined due to dual parallel inferior precentral sulci (black arrows)
Discussion
Left IFG has been proven not to be unique in speech function via positron emission tomography, magnetoencephalography, functional magnetic resonance imaging (fMRI) and especially electrical cortical stimulation (ECS) techniques in awake patients, lately. Broca’s area is considered to be involved in modulating the speech especially with bilateral dorsal premotor cortices [38]. A dual stream model consisting of a ventral and dorsal pathway for language processing is commonly accepted [6, 12, 13]. The dorsal stream is composed of white matter tracts connecting frontal lobe to the parietal and temporal lobes via arcuate fascicles, whereas the ventral stream connects these lobes via the external capsule. According to this model, different parts of Broca’s area have different roles in language processing [8, 13]. Nonetheless, Broca’s area is also considered to have a coordinator role in transformation of formation and reciprocal interactions between temporal and frontal cortices before articulation rather than producing the words [16].
The first postmortem study examining the anatomical asymmetry in Broca’s area was performed by Kononova [30], and the asymmetry of the PTR and POP was investigated in seven brains. Wada et al. [40] examined 100 adult and 85 infant brains and studied the left–right asymmetry of the PTR and POP by measuring the surface area of the gyri. Similarly, postmortem anatomical studies of Nikkuni et al. [33], Falzi et al. [15], Albanese et al. [1], Harasty et al. [25] are available. All of these studies aimed to determine the asymmetry of Broca’s area between the left and right hemispheres and often to prove asymmetry in favor of the left side; however, anatomical investigation was not the main purpose. In later years, for the same purpose, MRI-guided studies were published by Foundas et al. [17–21], Tomaiuolo et al. [39], Luders et al. [32], Keller et al. [26, 27] and more. Each of these studies detected major morphological variations in the sulcal structures defining the limits of the PTR and POP. The differences in the numbers of samples in different studies, the accepted limits for PTR and POP, the differences in nomenclature and the methods have led to a wide variety of results. Nonetheless, Amunts et al. demonstrated [3] great variability both in cytoarchitecture of Broadmann’s area 44 (POP) and 45 (PT) and the sulci of the IFG among their subjects. They have shown that areas 44 and 45 cannot be properly localized on MRIs because of the independent variability of sulcal pattern and the cytoarchitectonic borders of Broca’s area. However, voxel-based statistical analysis of Watkins et al. was negative for volume differences and asymmetry in Broca’s area [41].
The PS courses in front of the CS which is located between the precentral and postcentral gyri may consist of two to four segments. The vertical part that forms the posterior border of the POP is the first descending sulcus in front of the CS. In accordance with the literature, the two segment type was the most and the four segment type was the least frequent in both the left and right hemispheres in our study. As the IPS creates the posterior boundary of PTR [27, 34, 39], in the case of a rare dual parallel variation, this border can not be specified. In our study, we observed dual parallel IPS in 2 % (1/50) of the brains, bilaterally. We could not discover bilateral dual IPS rates in the literature.
In earlier studies, when the IPS is connected to the DS, an indirect connection between the IPS and SF was alleged to exist by Eberstaller and Cunningham [26, 27]. Moreover, POP may merge with the central gyrus, but this connection may be too medial to be seen in some brains. As a result, there may not always be a visible cortex bridge between the IPS and SF on the lateral surface; therefore, when the DS connects with the IPS or IFS, some observations may have confused the IPS with the DS. In light of previously described definitions, we distinguished the DS from the IPS with great attention. We did not observe a DS–IPS connection in our research, and at the same time, we demonstrated that the IPS never flows into the SF, even in the dual parallel case.
The IPS pattern results of our study are similar with the results of Ono’s study [34]. We found the ‘arcuat form’ at the highest rate in the right (29.8 %); and the ‘ramified form’ in the left (31.3 %). Likewise, in both the left (12.5 %) and the right (21.3 %), we detected the ‘Y’ shaped IPS the least.
The IFS is identifiable as the first ventral horizontal sulcus extending to the IPS on the lateral plane. Its length, continuity and connections are highly variable among brains [22, 34]. IFS was identified as a single, continuous segment in 56 % of the right and 40 % of the left, while the remainder consisted of two, three or four segments in Ono’s study [34]. The FMS and FOS lie on the anterior and ventral margin of the IFS. The FMS is the most anterior sulcus on the basal face. The FOS is located anteriorly and superiorly to the orbital sulci (Fig. 1). Petrides and Pandya [35] stated that these sulci could be seen as an additional anterior extension of IFS and showed that a misclassification was made by Ono et al. [34], and Keller et al.’s [26] study supported this claim. In the same study, they detected the IFS as a single, continuous segment in 54 % of the right and 50 % of the left hemispheres. Our findings support Keller’s et al. [26] study but are not in concordance with the results of Ono et al. [34]. This difference may be due not only to the misdiagnosis of these sulci but also differences in determining the number of segments of the IFS between studies. We distinguished the FOS and FMS from the IPS, and therefore, our IFSs were not more than two segments.
The connections between the IFS and PS are divided into three groups [22, 27, 34]:
-
1.
True connection: IFS was continuous with PS;
-
2.
Pseudoconnection: IFS seemed to be in a relationship with PS at the surface, but a merged bridge of cortex intersected this connection;
-
3.
No connection: IPS was not in a relationship with PS.
‘True connection’ is divided into two subgroups [26, 34]:
-
1A,
True-long connection: the IFS was continuous as a long, single sulcus;
-
1B,
True-short connection: the IFS consisted of a few short, discontinuous segments.
We could not demonstrate ‘pseudoconnection’ in our specimens. In Ono et al. [34] and Keller’s et al. [26] studies, on each side and in more than half of the samples, the IFS was detected as a single, continuous segment forming a ‘true long’ connection with IPS. On the lateral superficial view, because it is difficult to determine a hidden cortical bridge under a sulcus, we believe that the ‘pseudoconnection’ detected in Ono’s et al. [34] study might be a misdiagnosis and could be a branch of the IPS, but it was not schematized by the writer. We think that coronal sections demonstrating the intrasulcal structure or MRI-guided orthogonal sections are required to prove such a connection. While we did not demonstrate HR connection with the IFS, Keller et al. [26] did not encounter connections of IFS with HR or AR. They reported diagonal sulcus connection at 2 % in the right and 8 % in the left, which had values of 12 and 8 %, respectively, in our study.
Diagonal sulcus was demonstrated in 20 % of the right and 52 % of the left hemispheres by Keller et al. [26, 27]; 64 and 72 %, respectively, by Ono et al. [34]. Tomaiuolo et al. [39] reported the presence of DS in 32 % of the right and 34 % of the left hemispheres. Amunts [3] observed DS in 40 % of the right and 60 % of the left hemispheres. According to Knaus et al. [28, 29], these results were 38.3 and 30 %, respectively. We observed the DS in 48 % (24/50) of the right and 54 % (27/50) of the left hemispheres. The acceptance of the inferior end of the PS as a ‘diagonal sulcus’ by mistake in certain brains in Ono’s study [34] and the different sample sizes might be the cause of the discordant results between our studies. In addition to a non-unique morphology, the complexity of the connections with the neighboring sulci and differences between definitions of the DS may have caused it to be misclassified by different authors.
The connection of the DS with the AR was detected in 28 % and with the IPS in 4 % of the left hemispheres in Ono’s study [34]. The writers could not demonstrate these connections in the right hemispheres. In the same study, they found DS to be connected with the IFS in 4 % of the right and 24 % of the left hemispheres. In Keller’s et al.’s [26] study, the DS was connected with the AR in 4 % of the right and 8 % of the left hemispheres. The connection with IFS was determined to occur in 2 % on the right and 8 % on the left, in the same study. The DS was mostly found to be connected with the AR, on both sides. In Ono’s study [34], the writers found mostly the DS to be connected to the IPS on the right and to the AR on the left. In Keller’s study [26], the AR and IPS were most commonly connected with the DS on the right and the IPS on the left. We did not observe any connection with the IPS. The pronounced distinctions between the studies may be mostly due to the differences in the criteria for determining the DS. In our study, we also examined the bilateralism of the connections and observed the connection between DS and AR to be the most frequent.
Ascending ramus arises from SF as a deep and vertical ramus in front of DS when present. Rarely, it may be hidden in the IPS [39]. It was not documented in Ono’s study [34]. Tomaiuolo et al. [39] reported the AR to be absent in 1.9 % of the brains. Our results are compatible with the results of Keller et al. [26] and Tomaiuolo et al. [39]. We also demonstrated AR bilateralism and did not find such a result in the previous literature.
In the absence of HR, the front border of the PTR is indeterminable (Fig. 6b). We demonstrated HR in 94 % of the right and 96 % of the left hemispheres. It was absent in 2 % of the brains, bilaterally. Absent AR and HR were not defined in Foundas’ et al.’s [17–21] studies. Our results are compatible with the findings of Keller, who proved by orthogonal sections that these small rami may be absent in a very small percentage of brains [26, 27].
Wada et al. [40] measured the surface area of the posterior part of the PTR and POP and named this region the ‘frontal operculum’. In this study, the authors accepted the IPS as the posterior and the IFS as the dorsal boundary. They also stated that the rostral boundary of the frontal operculum is restricted by a deep sulcus, and in later studies, authors claimed that this referring sulcus was the ‘triangular sulcus’. Likewise, the PTR was interrupted by a descending branch of the IFS superiorly [37], which was termed the ‘inferior extension of the inferior frontal gyrus’ in our study. It was previously pronounced ‘frequent’, and we did not encounter the ratio of the presence of this sulcus in the literature. However, the TS and iIFS, also described by Ribas [37], were distinguished from each other in this study. The TS was located in the PTR and had no connection with IFS. We demonstrated that either TS or iIFS must be different sulci that can exist simultaneously in the same brain. We think that neither TS nor iIFS is a reliable rostral border of the frontal operculum, since either of them may not exist in every hemisphere.
The relationship between AR and HR presents certain variations. Ayberk et al. [5] classified PTR as ‘V’, ‘Y’ or ‘U’ shaped according to the relationship of HR with AR. Ono et al. [34] classified them as ‘separated’ or ‘common trunk and then separated.’ PTR takes the ‘V’ form when AR and HR arise from the same point of the SF as separate sulci; it takes the ‘U’ shape when they arise from different points of the SF and the ‘Y’ shape when they arise with a common trunk and then separate, as described in Ayberk’s study [5]. The ‘U’ and ‘V’ shapes of Ayberk et al. [5] correspond to the ‘separated rami’ and the ‘Y’ shapes to the ‘common trunk that separates afterwards’ of Ono et al. [34]. In both our study and Ayberk’s, ‘V’ shaped on the right and ‘Y’ shaped on the left were detected at the highest rates. We found ‘U’ shaped more and ‘Y’ shaped less often on either side compared to Ayberk’s study [5]. The difference might be due to the different sample sizes of the two studies.
In our study, we detected great variation and much morphological diversity between the right and left hemispheres in terms of each of the sulcal structures and their connections with the peripheral sulci. Interpersonal shape, length and quantitative variations of the sulcal contours participating in the structure of these regions are the causes of morphological asymmetry in different brains. The low rate of the ‘bilaterality’ property, which has not been examined in most of the previous studies, is an indicator of the low likelihood of the two hemispheres of the same brain having the same structure, number and connections of the sulcal and gyral structures.
Limitations of the study
Being a topographical examination is the main weakness of this study. Because approximately about two-thirds of the cerebral cortex is hidden in the sulci, studies not considering the intrasulcal anatomy might miss these hidden gyri [40]. The large number of samples and great effort to analyze the literature in the evaluation of the descriptions, to avoid a misdiagnosis according to the nomenclature, are the main strengths of our study. To our knowledge, this study has the second largest sample size in the past 40 years for the methodology reported.
Conclusion
At the present time, awake craniotomy with the aid of simultaneous ECS is a popular method of surgeries when the eloquent areas are involved. While the major concerns are decreasing the probability of permanent postoperative deficits and increasing the extent of resection, intraoperative mapping in awake patients also guides neuroscientists and neurosurgeons for a comprehensive insight into the complex functional anatomy of the brain. Combination and evaluation of preoperative functional imaging methods, intraoperative brain mapping with ECS in awake patients and postmortem anatomical studies may further lead us in comprehending the causes and importance of major anatomic variations.
References
Albanese E, Merlo A, Albanese A, Gomez E (1989) Anterior speech region. Asymmetry and weight–surface correlation. Arch Neurol 46(3):307–310
Alexander MP, Naeser MA, Palumbo C (1990) Broca’s area aphasias: aphasia after lesions including the frontal operculum. Neurology 40:353–362
Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412:319–341
Ardila A, Bernal B, Rosselli M (2016) How localized are language brain areas? A review of Brodmann areas involvement in language. Arch Clin Neuropsychol 31:112–122
Ayberk G, Yaglı E, Comert A, Esmer AF, Canturk N, Tekdemir I, Dinc H (2012) Anatomic relationship between the anterior sylvian point and the pars triangularis. Clin Anat 25:429–436
Axer H, Klingner CM, Prescher A (2013) Fiber anatomy of dorsal and ventral language streams. Brain Lang 127:192–204
Benson DF, Ardila A (1996) Aphasia: a clinical perspective. Oxford University Press, New York, pp 127, 135, 168, 169
Benzagmout M, Gatignol P, Duffau H (2007) Resection of World Health Organization Grade II gliomas involving Broca’s area: methodological and functional considerations. Neurosurgery 61(4):741–752
Bernal B, Ardila A, Rosselli M (2015) Broca’s area network in language function: a pooling-data connectivity study. Front Psychol 6:687
Broca P (1861) Nouvelle observation aphémie produite par un lésion de la moité postérieure des deuxième et troisième circonvolutions frontales. Bulletins de la Société Anatomique 6:398–407
Broca P (1861) Remarques sur le siége de la faculté du langage articulé, suivies d’une observatoin d’aphémie (Perte de la Parole). Bulletin de la Société Anatomique de Paris 6:330–357
Dick AS, Bernal B, Tremblay P (2014) The language connectome: new pathways, new concepts. Neuroscientist 20:453–467
Duffau H, Moritz GS, Mandonnet E (2014) A re-examination of neural basis of language processing: proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain Lang 131:1–10
Duvernoy H (2006) Atlas of morphology and functional anatomy of the brain. Springer, Berlin, pp 17–121
Falzi G, Perrone P, Vignolo LA (1982) Right–left asymmetry in anterior speech region. Arch Neurol 39(4):239–240
Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, Crone NE (2015) Redefining the role of Broca’s area in speech. Proc Natl Acad Sci USA 112(9):2871–2875
Foundas AL, Leonard CM, Heilman KM (1995) Morphologic cerebral asymmetries and handedness. The pars triangularis and planum temporale. Arch Neurol 52:501–508
Foundas AL, Leonard CM, Gilmore RL, Fennell EB, Heilman KM (1996) Pars triangularis asymmetry and language dominance. Proc Natl Acad Sci USA 93:719–722
Foundas AL, Eure KF, Luevano LF, Weinberger DR (1998) MRI asymmetries of Broca’s area: the pars triangularis and pars opercularis. Brain Lang 64:282–296
Foundas AL, Bollich AM, Corey DM, Hurley M, Heilman KM (2001) Anomalous anatomy of speech–language areas in adults with persistent developmental stuttering. Neurology 57:207–215
Foundas AL, Weisberg A, Browning CA, Weinberger DR (2001) Morphology of the frontal operculum: a volumetric magnetic resonance imaging study of the pars triangularis. J Neuroimaging 11:153–159
Germann J, Robbins S, Halsband U, Petrides M (2005) Precentral sulcal complex of the human brain: morphology and statistical probability maps. J Comp Neurol 493:334–356
Gil RS, Gatignol P, Capelle L, Mitchell MC, Duffau H (2005) The role of dominant striatum in language: a study using intraoperative electrical stimulations. J Neurol Neurosurg Psychiatry 76(7):940–946
Hagoort P (2006) On Broca, brain and binding. In: Grodzinsky Y, Amunts K (eds) Broca’s region. Oxford University Press, Oxford, pp 242–253
Harasty J, Double KL, Halliday GM, Kril JJ, McRitchie DA (1997) Language-associated cortical regions are proportionally larger in the female brain. Arch Neurol 54:171–176
Keller SS, Highley JR, Garcia-Finana M, Sluming V, Rezaie R, Roberts N (2007) Sulcal variability, stereological measurement and asymmetry of Broca’s area on MR images. J Anat 211:534–555
Keller SS, Crow T, Foundas A, Amunts K, Roberts N (2009) Broca’s area: nomenclature, anatomy, typology and asymmetry. Brain Lang 109:29–48
Knaus TA, Bollich AM, Corey DM, Lemen LC, Foundas AL (2006) Variability in perisylvian brain anatomy in healthy adults. Brain Lang 97:219–232
Knaus TA, Corey DM, Bollich AM, Lemen LC, Foundas AL (2007) Anatomical asymmetries of anterior perisylvian speech–language regions. Cortex 43:499–510
Kononova EP (1949) The frontal region. Architectonics of the cerebral cortex. Medgiz, Moscow, pp 309–343
Lemaire JJ, Golby A, Wells WM III, Pujol S, Tie Y, Rigolo L, Yarmarkovich A, Pieper S, Westin CF, Jolesz F, Kikinis R (2013) Extended Broca’s area in the functional connectome of language in adults: combined cortical and subcortical single-subject analysis using fMRI and DTI tractography. Brain Topogr 26:428–441
Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Toga AW (2006) Hemispheric asymmetries in cortical thickness. Cereb Cortex 16:1232–1238
Nikkuni S, Yashima Y, Ishige K, Suzuki S, Ohno E, Kumashiro H (1981) Left–right hemispheric asymmetry of cortical speech zones in Japanese brains. No to Shinkei 33(1):77–84
Ono M, Kubik S, Abernathey CD (1990) Atlas of the cerebral sulci. Thieme, New York
Petrides M, Pandya DN (2004) The frontal cortex. The human nervous system. Elsevier Academic Press, San Diego, pp 950–972
Plaza M, Gatignol P, Leroy M, Duffau H (2009) Speaking without Broca’s area after tumor resection. Neurocase 15(4):294–310
Ribas GC (2010) The cerebral sulci and gyri. Neurosurg Focus 28:E2
Tate MC, Herbet G, Moritz GS, Tate JE, Duffau H (2014) Probabilistic map of critical functional regions of the human cerebral cortex: Broca’s area revisited. Brain 137:2773–2782
Tomaiuolo F, MacDonald JD, Caramanos Z (1999) Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur J Neurosci 11:3033–3046
Wada JA, Clarke R, Hamm A (1975) Cerebral hemispheric asymmetry in humans. Cortical speech zones in 100 adults and 100 infant brains. Arch Neurol 32:239–246
Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC (2001) Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex 11:868–877
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Rights and permissions
About this article
Cite this article
Eser Ocak, P., Kocaelı, H. Investigation of topographical anatomy of Broca’s area: an anatomic cadaveric study. Surg Radiol Anat 39, 357–365 (2017). https://doi.org/10.1007/s00276-016-1748-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-016-1748-0