Abstract
Purpose
The aim of this study was to identify variations in the anatomy of the distal vertebral artery (VA) and posterior inferior cerebellar artery (PICA) with computed tomography (CT) angiography.
Methods
CT angiography was performed at two hospitals. And the results were analyzed for VA anomalies.
Results
Seven of the 3067 patients who received brain CT angiography in first hospital had seven intracranial VA fenestrations. Twelve of 546 patients who received CT angiography of intracranial and extracranial vessels in second hospital had 16 anatomical variations of the V3 segment. Two fenestrations of the V3 segment, three C1 origins of the PICA, seven aberrant VAs with an intradural course at the C2 level without a normal VA, and four aberrant VAs with an intradural course at the C2 level with a normal VA were observed. Seventeen of the 314 patients who received cervical CT angiography in second hospital had 21 anatomical variations of the VA. Two fenestrations of the V3 segment, six C1 origins of the PICA, three C2 origins of the PICA, one VA origin of the occipital artery, one fenestration of the V4 segment, five aberrant VAs with an intradural course at the C2 level without a normal VA, and three aberrant VAs with an intradural course at the C2 level with a normal VA were observed.
Conclusions
A certain number of anatomical variants of the distal VA and PICA may reflect variations in size and connections of the lateral or posterior spinal artery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anatomical variations commonly involve the distal segments (V3 and V4) of the vertebral artery (VA). The incidence and clinical significance of these variations have been reported in several studies, and various embryological explanations to explain the anomalous V3 and V4 segments have been proposed. The embryological basis for intracranial VA fenestrations is consistent with persistent vertebrobasilar anastomoses beyond the fifth week of intrauterine life [11]. Plexiform and longitudinal anastomotic connections develop from the cervical intersegmental arteries (CIAs) that are formed out of the primitive dorsal aorta. Fenestrations of the extracranial VA arise as a result of disorder during the transformation of plexiform anastomoses into a single vascular channel [18].
There are two embryological explanations for VA variations at the C1–2 level: an aberrant intradural VA at the C2 level with or without a normal VA and an extracranial origin of the posterior inferior cerebellar artery (PICA). The first explanation was suggested by Lasjaunias et al. [8] and Siclari et al. [14]. Lasjaunias et al. [8] proposed this complex anatomy of the distal VA after finding that a certain number of anatomical variants can be understood as segmental caliber variations of the distal VA and of one of its branches, the lateral spinal artery (LSA). Later, Siclari et al. [14] proposed that these variants are the result of variations in the development of the posterior spinal artery (PSA).
Another opinion was proposed by Uchino et al. [23] and Tokuda et al. [19]. According to Uchino et al. [23], if the first intersegmental artery (FIA) and the normal VA branch both persist, one branch of the VA takes the typical anatomical pathway, whereas the other branch enters the spinal canal between C1 and C2, resulting in an aberrant intradural VA at the C2 level with a normal VA. Uchino et al. [23] also proposed that if the FIA persists and continues to the PICA without fusion with the VA, it forms the extracranial C1/2 origin of the PICA.
Here, I describe a series of anatomical variants of the distal VA and PICA, and I review the embryological basis and anatomy of these variants.
Materials and methods
Images obtained by computed tomography (CT) angiography were analyzed for the presence of developmental anomalies of the distal VA and PICA. We excluded CT angiography performed on foreign patients or that obtained outside the two hospitals involved in this study. The CT angiographic studies were performed for a variety of clinical reasons, including symptoms of cerebral ischemia, hemorrhagic contusion, intracerebral hemorrhage, headache, dizziness, and routine check-up. All images were evaluated by one neurosurgeon.
Brain CT angiography of intracranial vessels performed at the first hospital
Brain CT angiography was performed in 3067 patients (1452 females, 1615 males; age range 9–102 years) between January 2011 and August 2013 in the first hospital, and the results were analyzed. CT angiography was performed using a Toshiba CT scanner (Toshiba Aquilion TSX-101A 64 Channel, Toshiba Medical Systems Corp., Tokyo, Japan). After the acquisition of the nonenhanced CT (NECT) data, contrast-enhanced CT angiography was performed. The parameters for the CT angiographic acquisition were as follows: 120 kVp, 300 mAs, field of view (FOV) 220 mm, detector collimation 64 × 0.5 mm, table speed 16 mm/rotation, gantry rotation speed 0.5 s/rotation, reconstructed section thickness 0.5 mm, and reconstruction increment 0.3 mm. The scan range extended from the foramen magnum to a point 1 cm above the level of the lateral ventricles. A total of 100 mL of iohexol (Iobrix® 350, Taejoon Pharmaceuticals Co. Ltd., Seoul, Korea) was administered intravenously using a power injector at a rate of 4.0 mL/s via an 18-gauge catheter positioned in a peripheral vein, and the scan delay was individually adapted using a bolus-tracking technique. For the bolus tracking, first, a single nonenhanced low-dose scan at the level of the upper neck was obtained. From the start of contrast material administration, repeated low-dose monitoring scans were obtained every second. When the first contrast was seen in the common carotid artery, the CT angiography was triggered automatically without any time delay. The data were transferred to a personal computer. Three-dimensional (3D) reconstructions of the images were performed using commercially available software (AquariusNET Viewer V4.4.6.74, TeraRecon Inc., San Mateo, CA, USA). From the data, 3D CT angiography images were reconstructed using a volume-rendering technique. A series of ten projection images at every 20° around the cephalocaudal axis and another ten images around the right–left axis were generated and then transferred to the picture archiving and communication system (PACS). We reviewed only anatomical variations of the V4 segment.
CT angiography of the intracranial and extracranial vessels performed at the second hospital
CT angiography of the intracranial and extracranial vessels was performed in 546 patients (297 females, 249 males; age range 14–94 years) between April 2013 and January 2015 at the second hospital, and the results were analyzed. An Aquilion Prime 160-slice CT scanner was used in 395 patients, and an Aquilion CXL edition 128-slice CT scanner (both instruments from Toshiba Medical Systems Corp.) was used in 151 patients. After the acquisition of the NECT data, contrast-enhanced CT angiography was performed. The parameters for the CT angiographic acquisition were as follows: 100 kVp, 225 mAs, FOV 220 mm, detector collimation 80 × 0.5 mm, table speed 25.5 mm/rotation, gantry rotation speed 0.75 s/rotation, reconstructed section thickness 0.5 mm, and reconstruction increment 0.3 mm for the Aquilion Prime and 120 kVp, 250 mAs, FOV 240 mm, detector collimation 64 × 0.5 mm, table speed 20.5 mm/rotation, gantry rotation speed 0.5 s/rotation, reconstructed section thickness 0.5 mm, and reconstruction increment 0.5 mm for the Aquilion CXL. The scan range extended from 2 cm below the aortic arch to a point 1 cm above the level of the lateral ventricles.
Cervical spinal CT angiography performed at the second hospital
Cervical spinal CT angiography was performed in 314 patients (141 females, 173 males; age range 7–86 years) between November 2008 and May 2015 in the second hospital, and the results were analyzed. The Aquilion Prime 160-slice CT scanner was used in 259 patients and the Aquilion CXL edition 128-slice CT scanner (both instruments from Toshiba Medical Systems Corp.) was used in 55 patients. After the acquisition of the NECT data, contrast-enhanced CT angiography was performed. The parameters for the CT angiographic acquisition were as follows: 120 kVp, 150 mAs, FOV 140 mm, detector collimation 80 × 0.5 mm, table speed 25.5 mm/rotation, gantry rotation speed 0.6 s/rotation, reconstructed section thickness 0.5 mm, and reconstruction increment 0.4 mm for the Aquilion Prime and 120 kVp, 300 mAs, FOV 220 mm, detector collimation 64 × 0.5 mm, table speed 20.5 mm/rotation, gantry rotation speed 0.5 s/rotation, reconstructed section thickness 0.5 mm, and reconstruction increment 0.4 mm for the Aquilion CXL. The scan range extended from 2 cm below the aortic arch to the sphenoid sinus.
CT angiography of the intracranial and extracranial vessels and cervical spinal CT angiography were performed at the second hospital, and the results were analyzed using the following method. A total of 100 mL of iopamidol (Pamiray® 370, Dongkook Pharmaceuticals Co., Seoul, Korea) was administered intravenously using a power injector at a rate of 4.0 mL/s via an 18-gauge catheter positioned in a peripheral vein, and the scan delay was individually adapted using a bolus-tracking technique. For the bolus tracking, first, a single nonenhanced low-dose scan at the level of the upper neck was obtained. With the start of contrast material administration, repeated low-dose monitoring scans were obtained every second. When the first contrast was seen in the common carotid artery, the CT angiography was triggered automatically without any time delay. The data were transferred to a personal computer. 3D reconstructions of the images were performed using commercially available software (Vitrea 2, Vital Images, Minnetonka, MN, USA). From the data, 3D CT angiography images were reconstructed using a volume-rendering technique. A series of 17 projection images at every 20° around the cephalocaudal axis were generated and then transferred to the PACS. We reviewed only anatomical variations of the V3 and V4 segments.
Fenestration of the VA that spans the origin of the PICA can be difficult to differentiate from a double origin of the PICA. For the purpose of classification, we divided these variations according to the classification proposed by Uchino et al. [21]. In cases of a PICA of double origin, the distal segment of the PICA is larger than the proximal two channels. By contrast, a PICA arising from the VA fenestration has a large caudal channel.
Results
CT angiography of intracranial vessels performed at the first hospital
Seven (four females, three males: age range 24–86 years) of the 3067 patients who received CT angiography had seven intracranial VA fenestrations (Figs. 1, 2). Five right VAs and two left VAs were anomalous.
a Computed tomography angiography shows a small fenestration in the right intracranial vertebral artery (red arrow fenestration). b Computed tomography angiography demonstrates a large fenestration in the left intracranial vertebral artery (red arrow fenestration). c Computed tomography angiography shows a large fenestration in the right intracranial vertebral artery (VA), from which the posterior inferior cerebellar artery (PICA) arises (red arrowhead PICA). The possible direction of blood flow (red arrow) in the cranial channel (black arrowhead) flows toward the terminal segment of the VA because the caudal channel (blue arrowhead) is larger than the PICA
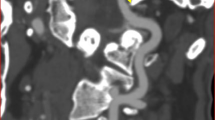
Computed tomography angiography shows a large fenestration in the right vertebral artery (VA), from which the posterior inferior cerebellar artery (PICA) arises (red arrowhead PICA). The proximal point of the VA fenestration is located in the extracranial VA. The possible direction of blood flow (black arrow) in the cranial channel (black arrowhead) flows toward the terminal segment of the VA because the caudal channel (yellow arrowhead) is larger than the PICA
CT angiography of intracranial and extracranial vessels performed at the second hospital
Twelve (eight females, four males; age range 32–88 years) of the 546 patients who received CT angiography had anatomical variation of the V3 segment. Twelve patients had 16 V3 anomalies (four right side and 12 left side). Two fenestrations of the V3 segment (Fig. 3), three C1 origins of the PICA (one PSA type and two LSA type) (Fig. 4), seven aberrant VAs coursing intradurally at the C2 level without a normal VA (Figs. 3, 5), and four aberrant VAs coursing intradurally at the C2 level with a normal VA (Fig. 6) were observed.
Antero-posterior view (a) and lateral view (b) of bilateral C1 origin of the posterior inferior cerebellar artery (PICA). The left PICA (yellow arrow) shows the posterior position of the PICA at the level of the foramen magnum and a hairpin turn of the proximal segment of the PICA. These features are consistent with a posterior spinal artery (PSA). The right PICA (red arrow) crosses the foramen magnum in a more anterior portion, and its position is consistent with the lateral spinal artery (LSA) rather than the PSA. The right PICA is LSA type
Cervical spinal CT angiography performed at the second hospital
Seventeen of the 314 patients (nine females, eight males; age range 22–62 years) who received cervical spinal CT angiography had 21 anatomical variations of the VA (11 right side and ten left side). Two fenestrations of the V3 segment (Fig. 7), six C1 origins of the PICA (Fig. 8), three C2 origins of the PICA (Fig. 9), one VA origin of the occipital artery, one fenestration of the V4 segment (Fig. 10), five aberrant VAs coursing intradurally at the C2 level without a normal VA, and three aberrant VAs coursing intradurally at the C2 level with a normal VA were observed.
Computed tomography angiography shows a large fenestration in the right vertebral artery (VA), from which the posterior inferior cerebellar artery (PICA) arises (black arrowhead PICA). The possible direction of blood flow (black arrow) in the cranial channel (yellow arrowhead) flows toward the terminal segment of the VA because the caudal channel (red arrowhead) is larger than the PICA. The right VA is aberrant VA with an intradural course at the C2 level
Discussion
Developmental anatomy of the VA
According to Padget [11], development of the VA in the human embryo occurs during the 7- to 18-mm embryonic stage (32–40 days). The VA does not develop as a proper artery possessing its own specific territory; rather, it results from longitudinal anastomoses between CIAs [24]. According to the description of Padget [11], on the 32nd day of the embryonic period, plexiform and longitudinal anastomotic connections develop from the CIAs that are formed out of the primitive dorsal aorta. After that, with the continuing transformation of the anastomoses to a single lumen, the primitive VA forms. At the same time, the first six CIAs, but not the seventh, undergo regression. The seventh CIA forms the proximal part of the subclavian artery, which also includes the origin of the VA. The proatlantal intersegmental artery (PIA) is the most cranial of the seven CIAs found in very early embryos. The carotid end of this artery, like those of the lower six, normally dwindles as the VA is formed, although the larger part of this artery persists as the horizontal suboccipital segment of the VA [9].
Embryological explanation for VA fenestrations
Unfortunately, the terms “fenestration” and “duplication” are used inconsistently throughout the literature and are often incorrectly used synonymously. The term “duplication” is appropriately defined by a VA with two origins and a variable course and fusion [3]. By contrast, “fenestration” represents a vessel with a single origin that divides anywhere along its course into parallel segments that may lie within or outside the vertebral canal [3]. Fenestrations of the VA can be situated either at the intracranial or extracranial segments of the VA. Extracranial VA fenestrations are rarely found below the C2 level [15].
The first CIA anastomosis that appears temporarily between the basilar artery and the VA is termed the primitive lateral vertebrobasilar anastomosis [2, 7]. The embryological basis for intracranial VA fenestration is consistent with persistent vertebrobasilar anastomoses beyond the fifth week of intrauterine life and which are the intracranial equivalent of longitudinal neural arteries [11] and which form the primitive intradural, segmental, and intersegmental arteries of the posterior fossa [13]. In this article, the anomalous arteries shown in Fig. 1a–c are examples of intracranial fenestration of the VA.
According to the description of Padget [11], on the 32nd day of the embryonic period, plexiform and longitudinal anastomotic connections develop from the CIAs formed from the primitive dorsal aorta. Fenestrations of VA arise as a result of disorder during the transformation of plexiform anastomoses into a single vascular channel [18]. In this article, the anomalous arteries shown in Figs. 3 and 7 are examples of extracranial fenestration of the VA.
The fenestration of the intracranial VA is frequently large, as shown in the anomalous vessels in Figs. 2 and 10, and the PICA sometimes arises from the fenestrated segment. This large fenestration of VA was reported by Uchino et al. [22, 24]. Large fenestrations of the intracranial VA may form because of persistence of the anterior arterial collateral system [6]. This large fenestration of the VA has similar angiographic features compared with the double origin of the PICA. Therefore, this large fenestration of the VA also may result from anastomoses between the LSA and PICA.
Embryological mechanism for an aberrant VA with an intradural course at the C2 level
Researchers have suggested two possible developmental explanations for an aberrant VA. One is a persistent FIA [19, 23]. If the FIA persists without persistence of the normal VA branch, the VA takes an anomalous course that enters the spinal canal at the level between the C1 and C2 vertebral bodies [19, 23]. Lasjaunias et al. [8] and Siclari et al. [14] suggested a second mechanism. These authors showed that the intradural course of the distal VA at the C2 level is related to variations in the size and connection of the LSA or PSA. Lasjaunias et al. [5] proposed that, at the upper cervical level, the so-called intradural course of the VA at the C2 level corresponds most often to an enlarged LSA.
The PIA is the most cranial of the seven CIAs found in very early embryos. The carotid end of this artery, like those of the lower six, normally dwindles as the VA is formed. However, the greater part of this artery persists as the horizontal suboccipital segment of the VA. If the carotid end of the PIA does not regress, a persistent PIA results. A persistent PIA arises from the internal carotid artery and runs to the point beyond which its course is indistinguishable from that of the normal VA [9]. A persistent CIA is a carotid vertebral anastomosis in the neck [9]. Persistent FIA was originally described as a persistent carotid connection. The segment of the VA proximal to a persistent FIA is generally absent [9]. Failure of involution in one of the first six CIAs (i.e., a persistent CIA) causes a variety of abnormal origins of the VA. If a persistent CIA occurs in the upper (first or second) CIAs, the result is an abnormal origin of the VA from the internal or external carotid artery; if it occurs in the lower (third through sixth) CIAs, the result is an abnormal origin of the VA from the aortic arch or the common carotid artery [1]. I could not identify any carotid vertebral anastomoses in the anomalous vessels shown in Figs. 5 and 6. CIAs are usually located in the extradural space. In Figs. 5 and 6, the anomalous VAs are located mainly in the intradural space, not the extradural space, although its origin is located in the extradural space.
Therefore, persistent FIA is a misnomer for describing the anomalous vessels shown in Figs. 5 and 6. An aberrant VA coursing intradurally at the C2 level is a more precise term for describing the abnormal vessels shown in Figs. 5 and 6.
The CIAs that anastomose to produce the VA probably also form the vertebral radicular spinal arteries. These vessels enter the neural foramina at every cervical level to supply the intraspinal plexus. Vertebral radicular arteries, which have been described as LSAs by Lasjaunias et al. [8] and Siclari et al. [14], are located in the intradural space. Therefore, these anomalous vessels are not FIAs but are LSAs or PSAs originating from the FIA.
Terminology to describe an aberrant VA with an intradural course at the C2 level with a normal VA
Several terms have been used to describe the VA anomaly shown in Fig. 6. These include a duplicated atlantoaxial portion of the VA [19], partial duplication of the distal VA above and below the C1 arch [19], fenestration of the VA [12, 26], fenestration of the VA above and below C1 [23, 25], persistence of the first FIA with a normal VA, duplication of the distal VA, and fenestration of the VA at the atlantoaxial junction [4].
The term “duplication” for this anomalous vessel is inappropriate because this vessel does not have two origins. I think that “a C2 segmental type of the VA associated with a normal VA”, or “an aberrant VA with an intradural course at the C2 level associated with a normal VA” are appropriate terms for describing the VA anomaly shown in Fig. 6.
Embryological mechanism to explain the extracranial origin of the PICA
Uchino et al. [23] proposed that, if the FIA persists and continues to the PICA without fusion with the VA, it forms the extracranial C1/2 origin of the PICA. However, as mentioned above, a persistent FIA that continues to the PICA without fusion with the VA cannot be an extracranial C1/2 origin of the PICA. Lasjaunias et al. [8] suggested that normal variation of the LSA causes a cervical vertebral origin of the PICA at C1 or C2. Siclari et al. [14] proposed that the C1 and C2 origins of the PICA result from variations in the development of the PSA or LSA. I agree with the opinion of Siclari et al. [14].
Importance of the LSA and PSA to distal VA and PICA anomalies
Lasjaunias et al. [8] proposed a schematic representation of 14 different arrangements of the vertebrobasilar system involving the LSA system. Later, Siclari et al. [14] described the detailed developmental anatomy of the LSA and PSA after a thorough analysis of cerebral angiography (especially for LSA and PSA). Siclari et al. [14] proposed a schematic representation of the normal anatomy of the LSA and PSA, as well as some of their variations (Fig. 11). Siclari et al. [14] also showed that the posterior location of the PICA at the level of the foramen magnum, hairpin turn of its proximal segment, and presence of a separate LSA help in identifying the PSA-type variant. In an anatomical report, Mercier et al. [10] reported a separate LSA in a case of a C1 origin of the PICA. Therefore, the C1 origin of both PICAs shown in Fig. 5 in the paper by Mercier et al. [10] are the PSA type.
Schematic representation of the normal anatomy of the posterior spinal artery (PSA) and lateral spinal artery (LSA) (a, d), as well as some of their variations (b, c, e, f). a The extradural and intradural segments of the vertebral artery (VA) are shown in black and dark gray, respectively. The PSA originates from the extradural VA and takes a parallel course before bifurcating into a descending posterolateral ramus (the PSA per se) and an ascending ramus that joins the posterior inferior cerebellar artery (PICA). The latter may become a variant origin of the PICA. The anterior spinal artery (ASA) is also shown. b The C1 origin of the PICA (PSA type). The anastomosis between the ascending ramus of the PSA and the PICA is prominent, and the PSA serves as the actual origin of the PICA. c A distal VA duplication of the PSA type, in which the true distal VA is paralleled by a supernumerary vascular segment that branches off the PICA. The relative size of the two limbs can vary. If the true distal VA is much smaller or absent, the variant becomes an aberrant course of the VA (PSA type). d The extradural and intradural segments of the VA are shown in black and dark gray, respectively. The LSA is the most lateral of the three cervical spinal axes (ASA, PSA, and LSA). It establishes several segmental connections with the VA (C1–C4) as well as a cranial anastomosis with the PICA. e The C1, C2, and C3 origins of the PICA (LSA type). The rostral anastomosis of the LSA with the PICA is prominent, and any of the lateral segmental connections of the LSA with the VA, alone or in combination, may serve as a PICA origin. f An aberrant VA with an intradural course at the C2 level with a normal VA of the LSA type. This time, the true VA is cranial to the supernumerary limb, which is made of a prominent LSA segment. If the true distal VA is much smaller or absent, the variant becomes an aberrant course of the VA (LSA type). Reproduced with permission from Siclari et al. [14]. For clarity, duplication of the distal VA of the LSA type of F in original article is modified to an aberrant VA with an intradural course at the C2 level with a normal VA of the LSA type
I also agree with the opinion of Siclari et al. [14]. The C1 origin of the left PICA shown in Fig. 4 here is the PSA type because of the posterior position of the PICA at the level of the foramen magnum and the hairpin turn of the proximal segment of the PICA.
Clinical significances of these anomalies
Some authors state that fenestration of the VA is an incidental finding and has no significant pathological and clinical results [3]. However, some other authors report that it increases the prevalence of aneurysm and vascular pathology [20].
The extracranial PICA is one of the cases that results in unpredictable anatomy and surgeons should be careful when confronted with these variations. The diagnosis of ruptured aneurysms of this area fail due to inadequate angiograms, and it should be kept in mind that such aneurysms are considered dangerous for endovascular treatment and pose hazards to the patient while attempting a lateral C1–C2 puncture [16].
Aberrant VA with an intradural course at the C2 level and extracranial origin of PICA are asymptomatic and found incidentally. But these vessels may cause cervical cord compression myelopathy, cervical pain, occipital neuralgia, and accessory nerve palsy [17]. If craniovertebral junction surgery is planned, preoperative identification of these anomalies is very important to avoid complications, such arterial injury, during surgical intervention [23].
Limitations of this study
We could not confirm the exact course of the PICA because of a narrow FOV and bony structures. Thus, the exact types (PSA or LSA type) of the C1 or C2 origin of the PICA in cervical spine angiography were indeterminable.
Conclusions
Anatomical variations commonly involve the distal segments of the VA and PICA. Several embryological mechanisms have been suggested to explain these variations, but I believe that the term “persistent FIA” has been used inappropriately. The fenestrations of the distal VA are linked to persistent vertebrobasilar anastomoses beyond the fifth week of intrauterine life and a result of disorder during the transformation of the plexiform anastomoses into a single vascular channel. A certain number of anatomical variants of the distal VA and PICA may reflect variations in the development of the LSA and PSA.
Abbreviations
- 3D:
-
Three-dimensional
- ASA:
-
Anterior spinal artery
- CIA:
-
Cervical intersegmental artery
- CT:
-
Computed tomography
- FIA:
-
First intersegmental artery
- FOV:
-
Field of view
- LSA:
-
Lateral spinal artery
- NECT:
-
Nonenhanced CT
- PACS:
-
Picture archiving and communication system
- PIA:
-
Proatlantal intersegmental artery
- PICA:
-
Posterior inferior cerebellar artery
- PSA:
-
Posterior spinal artery
- VA:
-
Vertebral artery
References
Chen CJ, Wang LJ, Wong YC (1998) Abnormal origin of the vertebral artery from the common carotid artery. AJNR Am J Neuroradiol 19:1414–1416
Hasegawa T, Ito H, Hwang WZ, Yamamoto S (1986) Single extracranial–intracranial duplication of the vertebral artery. Surg Neurol 25:369–372
Ionete C, Omojola MF (2006) MR angiographic demonstration of bilateral duplication of the extracranial vertebral artery: unusual course and review of the literature. AJNR Am J Neuroradiol 27:1304–1306
Koyanagi S, Shiraishi T, Ueta K, Tabuchi K (1991) Bilateral fenestrations of the vertebrobasilar artery with trigeminal neuralgia. Case report. Neurol Med Chir (Tokyo) 31:995–998
Lasjaunias P, Berenstein A, ter Brugge KG (2001) Surgical Neuroangiography. Clinical vascular anatomy and variations, 2nd edn. Springer, Berlin, pp 240–241
Lasjaunias P, Braun JP, Hasso AN, Moret J, Manelfe C (1980) True and false fenestration of the vertebral artery. J Neuroradiol 7:157–166
Lasjaunias P, Guibert-Tranier F, Braun JP (1981) The pharyngo-cerebellar artery or ascending pharyngeal artery origin of the posterior inferior cerebellar artery. J Neuroradiol 8:317–325
Lasjaunias P, Vallee B, Person H, Ter Brugge K, Chiu M (1985) The lateral spinal artery of the upper cervical spinal cord. Anatomy, normal variations, and angiographic aspects. J Neurosurg 63:235–241
Mayer PL, Kier EL (1993) The ontogenetic and phylogenetic basis of cerebrovascular anomalies and variants. In: Apuzzo ML (ed) Brain surgery. complication avoidance and management. Churchill Livingstone, New York, pp 709–760
Mercier PH, Brassier G, Fournier HD, Picquet J, Papon X, Lasjaunias P (2008) Vascular microanatomy of the pontomedullary junction, posterior inferior cerebellar arteries, and the lateral spinal arteries. Interv Neuroradiol 14:49–58
Padget DH (1948) The development of the cranial arteries in the numan embryo. Contrib Embryol 32:205–261
Salunke P, Futane S, Sahoo SK, Ghuman MS, Khandelwal N (2014) Operative nuances to safeguard anomalous vertebral artery without compromising the surgery for congenital atlantoaxial dislocation: untying a tough knot between vessel and bone. J Neurosurg Spine 20:5–10
Sanders WP, Sorek PA, Mehta BA (1993) Fenestration of intracranial arteries with special attention to associated aneurysms and other anomalies. AJNR Am J Neuroradiol 14:675–680
Siclari F, Burger IM, Fasel JH, Gailloud P (2007) Developmental anatomy of the distal vertebral artery in relationship to variants of the posterior and lateral spinal arterial systems. AJNR Am J Neuroradiol 28:1185–1190
Sim E, Vaccaro AR, Berzlanovich A, Thaler H, Ullrich CG (2001) Fenestration of the extracranial vertebral artery: review of the literature. Spine (Phila Pa 1976) 26:E139–E142
Tabatabai SA, Zadeh MZ, Meybodi AT, Hashemi M (2007) Extracranial aneurysm of the posterior inferior cerebellar artery with an aberrant origination: case report. Neurosurgery 61:E1097–E1098
Takahashi T, Tominaga T, Hassan T, Yoshimoto T (2003) Cervical cord compression with myelopathy caused by bilateral persistence of the first intersegmental arteries: case report. Neurosurgery 53:234–237
Tetiker H, Cimen M, Kosar MI (2014) Fenestration of the vertebral artery: case presentation. Folia Morphol (Warsz) 73:84–86
Tokuda K, Miyasaka K, Abe H, Abe S, Takei H, Sugimoto S, Tsuru M (1985) Anomalous atlantoaxial portions of vertebral and posterior inferior cerebellar arteries. Neuroradiology 27:410–413
Tran-Dinh HD, Soo YS, Jayasinghe LS (1991) Duplication of the vertebro-basilar system. Australas Radiol 35:220–224
Uchino A, Saito N, Ishihara S (2015) Double origin of the posterior inferior cerebellar artery diagnosed by MR angiography: a report of two cases. Neuroradiol J 28:187–189
Uchino A, Saito N, Okada Y, Kozawa E, Nishi N, Mizukoshi W, Inoue K, Nakajima R, Takahashi M (2012) Fenestrations of the intracranial vertebrobasilar system diagnosed by MR angiography. Neuroradiology 54:445–450
Uchino A, Saito N, Watadani T, Okada Y, Kozawa E, Nishi N, Mizukoshi W, Inoue K, Nakajima R, Takahashi M (2012) Vertebral artery variations at the C1–2 level diagnosed by magnetic resonance angiography. Neuroradiology 54:19–23
Uchino A, Sawada A, Takase Y, Kudo S (2002) Extreme fenestration of the right vertebral artery: magnetic resonance angiographic demonstration. Eur Radiol 12(Suppl 3):S32–S34
Wakao N, Takeuchi M, Nishimura M, Riew KD, Kamiya M, Hirasawa A, Kawanami K, Imagama S, Sato K, Takayasu M (2014) Vertebral artery variations and osseous anomaly at the C1–2 level diagnosed by 3D CT angiography in normal subjects. Neuroradiology 56:843–849
Yamazaki M, Okawa A, Furuya T, Sakuma T, Takahashi H, Kato K, Fujiyoshi T, Mannoji C, Takahashi K, Koda M (2012) Anomalous vertebral arteries in the extra- and intraosseous regions of the craniovertebral junction visualized by three-dimensional computed tomographic angiography: analysis of 100 consecutive surgical cases and review of the literature. Spine (Phila Pa 1976) 37:E1389–E1397
Acknowledgments
This work was supported by research Grant from a Pohang SM Christianity Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that he have no conflict of interest with any organization or institute.
Rights and permissions
About this article
Cite this article
Kim, M.S. Developmental anomalies of the distal vertebral artery and posterior inferior cerebellar artery: diagnosis by CT angiography and literature review. Surg Radiol Anat 38, 997–1006 (2016). https://doi.org/10.1007/s00276-016-1654-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-016-1654-5