Abstract
Purpose
To evaluate the ability of MDCT reformations in describing the celiac trunk vascular anatomy and variations.
Materials and methods
A total of 555 MDCT angiographies of the abdominal aorta performed between January 2002 and July 2005 were retrospectively reviewed to assess the celiac trunk vascular anatomy and variations. All the patients with pathological condition likely to affect normal vascular anatomy as well as CT exams technically inadequate were excluded from our study.
Results
A total of 524 MDCT angiographies of abdominal aorta were included in our study. The classical configuration of the celiac trunk was detected in 72.1%. The hepato-splenic trunk was detected in 50.4% of cases; the hepato-gastro-splenic trunk was detected in 19.4% of cases; the gastro-splenic trunk was detected in 2.3% of cases. The hepato-spleno-gastric trunk associated with hepatic arteries variants were found in 15.4%. The hepato-splenic trunk, the hepato-gastric trunk, the hepato-splenic-mesenteric trunk, and the spleno-gastric trunk were found in 2.7, 5, 0.4, and 3.6%, respectively. In 0.6%, we found an absent celiac trunk.
Conclusion
The knowledge of the type of anatomical variants and their subtypes is fundamental for a correct pre-operative vascular planning in surgical or radiological abdominal procedures. Multidetector-row CT (MDCT) provides high-quality 3D-reconstructed images and allows non-invasive assessment of normal anatomy and anatomic variants of celiac trunk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Celiac trunk anatomical variants are not infrequent and knowledge of the existing aberrations is becoming mandatory in planning and conducting surgical or interventional procedures. In detail, the success of liver transplantation surgery as well as fashioning intestinal anastomosis is strictly dependent on an adequate pre-operative vascular planning. Furthermore, the knowledge of exact anatomy has also consequences on hepatic arterial infusion chemotherapy via catheter for therapy of unresectable advanced liver malignancies [2, 5, 7, 16, 19].
Conventional angiography represents the gold standard of vessel imaging. However, although generally considered safe, DSA may be associated with complications that result from the procedure itself, with a complication rate of up to 1% depending on the experience of the operators, site of vascular access, diameter of the catheter, and administered contrast material [1, 4, 18].
Multidetector-row CT (MDCT) has brought significant advantages as compared to single detector-row CT (SSCT): the simultaneous acquisition of more sections during a complete rotation of the gantry, together with its increased rotational speed (0.5 s and less), lead to a decrease in the acquisition time, allowing the analysis of large anatomical volumes, with better spatial and temporal resolution of images, rationalizing the use of contrast medium [10, 12, 14].
Due to these advantages, MDCT angiography has become a valuable minimally invasive tool for the visualization of normal vascular anatomy and its variants, as well as pathological conditions of the celiac trunk and its vessels. Despite to DSA, MDCT allows in one step fashion to correctly assess both the abdominal arteries and parenchyma. Furthermore, reformatted three-dimensional MDCT images allow the visualization of vascular structures in angiography-equivalent planes other than the axial, useful for complex vascular anatomy to reduce the risk of iatrogenic injuries.
Therefore, it is important that surgeons, interventional radiologists, and also abdominal imagers be familiar with the full gamut of possible celiac trunk variants [3, 20].
The purpose of this article is to evaluate the ability of MDCT reformations in describing the celiac trunk vascular anatomy and variations.
Materials and methods
Patients
A computer search was performed to identify all the patients who had undergone MDCT angiography of the abdominal aorta in our department between January 2002 and July 2005.
A total of 555 CT angiographies of abdominal aorta of patients with suspected aneurysm was included in our study and retrospectively reviewed to evaluate the visibility of the celiac trunk and its branches. All the patients with pathological condition likely to affect normal vascular anatomy as well as CT exams technically inadequate were excluded from our study.
For all the CT examinations, a written informed consent was obtained.
Imaging technique
CT technique
CT scans were acquired on a 4-channel multidetector-row CT system (Somatom Plus Volume Zoom; Siemens Medical Systems, Forcheim, Germany). In all the patients unenhanced CT images were obtained from the level of the diaphragm to the symphysis pubis with 4 × 2.5-mm slice collimation, a pitch of 6, 5-mm slice width and reconstruction interval, a table speed of 15-mm/rotation and a 0.5 s gantry rotation time.
Contrast-enhanced images were performed from suprarenal abdominal aorta to the common femoral artery with 4 × 1-mm collimation, a pitch of 6, 1.25 mm slice width and 1-mm reconstruction interval, a table speed of 15-mm/rotation, 0.5 s rotation time, with 130 mAs and 120 KV.
Contrast-medium injection
In all the patients, 120 ml of iodinate non-ionic contrast medium (iomeprol 300 mgI/mL, Iomeron; Bracco, Milan, Italy) was injected into the brachial vein with a power injector at a flow rate of 3 ml/s. Scan delay was individualized per patient, using Siemens’s proprietary bolus-tracking software (C.A.R.E. Bolus), to capture 100 HU on the abdominal aorta, at the level of the celiac trunk, to trigger scanning and ensure a correct peak enhancement.
Image postprocessing
MDCT angiograms were evaluated and interactively processed on dedicated workstations to visualize the vascular structures from all points of view [13]. The goals of 3D-reconstructions (maximum-intensity projection, MIP; volume-rendering techniques, VRT) are to enhance detection of normal anatomy, anatomical variants, and pathologies, facilitating communication among clinicians. At our institution, we use a CT off-line workstation for image reconstruction (Leonardo, Siemens Medical Solutions, Forchheim, Germany).
Results
A total of 31 patients were excluded from our study; 22 of them due to the presence of pathological condition likely to affect normal vascular anatomy, such as previous surgical procedures. Nine MDCT exams were considered technically inadequate (0.02%) due to bad enhancement of arteries (4/555) or artifact movements (5/555).
A total of 524 MDCT angiographies of abdominal aorta were retrospectively reviewed.
According to the Michels classification [8] different types of normal anatomy or anatomic variants were described (Table 1).
Hepato-spleno-gastric trunk
This is the classical configuration of the celiac trunk, detected in a total of 378 patients of our series (72.1%). According to established classification, three subtypes can be described:
Hepato-splenic trunk
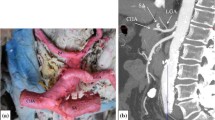
The common trunk origins from the aorta and ends dividing into common hepatic and splenic arteries. From common trunk arises the left gastric artery, the smallest celiac branch, which ascends cranially running along the gastric lesser curvature. This subtype was detected in 264 patients (50.4%, Fig. 1).
Hepato-gastro-splenic trunk
The common trunk, originating from the aorta, ends dividing into a trifurcation (left gastric, common hepatic, and splenic arteries). This subtype was detected in 102 patients (19.4%, Fig. 2).
Gastro-splenic trunk
This is a relatively rare classical subtype, detected in our series in only 12 patients (2.3%). Celiac trunk ends dividing into common hepatic and splenic arteries whereas the left gastric artery arises from splenic artery, which is dominant.
Hepato-spleno-gastric trunk + hepatic variants
The left gastric, common hepatic and splenic arteries arise from common trunk associated with: (1) the presence of a replaced right common hepatic artery arising from the abdominal aorta or the superior mesenteric artery, detected in 49 patients (9.3%, Fig. 3); (2) the presence of a replaced/accessory left common hepatic artery arising from the left gastric artery, detected in 31 patients (5.9%). A rare subtype of this variation (3), represented by the early bifurcation of a short common hepatic artery in right and left hepatic arteries with an accessory left hepatic artery arising from left gastric artery, was detected in one patient (0.2%, Fig. 4).
Hepato-splenic trunk
The common trunk ends dividing into common hepatic and splenic arteries whereas the left gastric artery arises from the aorta (14/524, 2.7%, Fig. 5). A rare subtype of this variation, detected in one patient (0.2%) of our series can be represented by the concomitant presence of a replaced right hepatic artery arising from the aorta (Fig. 6).
Hepato-gastric trunk
Common hepatic and left gastric arteries origin from common trunk whereas the splenic artery originates from the aorta (Fig. 7) or from the superior mesenteric artery (26/524, 5%).
Hepato-splenic-mesenteric trunk
The common hepatic, splenic, and superior mesenteric arteries form a single trunk whereas left gastric artery arises directly from the aorta (Fig. 8). This rare variation was detected in only two patients (0.4%).
Spleno-gastric trunk
This variation, detected in 19 patients (3.6%), was represented by the origin of splenic and left gastric arteries from a common artery with common hepatic artery arising from the aorta (Fig. 9) or from the superior mesenteric artery (Fig. 10).
Absent celiac trunk
The three main arteries arise directly from the abdominal aorta or from the superior mesenteric artery (Fig. 11) in three patients of our series (0.6%).
Discussion
Variant hepatic and celiac arterial anatomy have been reported in 55% of patients on the basis of initial cadaveric dissections by Michels [8]. In 1969, Redman and Reuter [11] reported “most of the variations” seen in 50% of the population “have little surgical significance”. However, today, because of the development of interventional and new surgical techniques to treat both primary and metastatic tumors and the increasing availability of living related liver transplant donors, the accurate depiction and definition of the hepatic arterial anatomy are important. It is also mandatory that interventional radiologists who perform hepatic arterial embolization be familiar with both common and rare hepatic arterial variants, because failure to recognize the presence of an aberrant vessel can result in incomplete embolization. So the presence of different arterial variants may alter patient management [2, 5, 7, 16, 19]. MDCT angiography is accurate and minimally invasive for the evaluation of the celiac trunk, allowing the description of normal anatomy as well as anatomical variants: it spares the patient the discomfort and the associated morbidity of angiography, analgesia, and postprocedural observation [9, 15].
The anatomical variations of the celiac trunk are due to developmental changes in the ventral splanchnic arteries. The embryological explanation by Tandler [17] was a longitudinal anastomosis (Laengsanastomose) between the four roots of the omphalomesenteric artery. The anatomizing vessel remains with the first root and gives rise to the hepatic, left gastric, and splenic arteries. On the other hand, separation from the fourth root gives rise to the superior mesenteric artery. If this separation takes place at a higher level, one of the branches is displaced to the superior mesenteric artery. Disappearance of the first or fourth root generates a common celiacomesenteric trunk. A hepato-spleno-mesenteric trunk may be due to an anomalous separation of the longitudinal anastomosis with the left gastric artery remaining with the first root, and the common hepatic and splenic arteries remaining with the fourth root [6].
For hepatic artery anatomical variations study, we followed Michels classification. We identified normal common hepatic artery in 72.1% of the patient (Michels I). In classic visceral anatomy, the celiac trunk originates from the abdominal aorta and gives origin to the left gastric artery, the splenic artery, and the common hepatic artery.
According to Michels classification, the most common variant observed was a replaced right hepatic artery originating from the superior mesenteric artery (Michels III), identified in 9.3% of patients. This frequency is similar to the results of the cadaveric study by Michels. It is importat to recognize a replaced right hepatic artery when performing pancreaticoduodenectomy and for porta hepatis dissection during hepatic resection. Therefore, if a head or uncinate process pancreatic cancer involves a replaced right hepatic, it precludes the patient from surgical resection. The second most common arterial variant identified was a replaced left hepatic artery originating from the left gastric artery, seen in 5.9% of patients (Michels II). The reported incidence of this variant is 10%, whereas the incidence in our study was lower [9]. It is important to detect this variant prior performing left hepatectomy because this vessel must be identified and ligated; the knowledge of this variant facilitates portal dissection because the major arterial branch to the left liver does not need to be found in the porta hepatis. The third most common arterial variant observed was the common hepatic artery arising from the superior mesenteric artery, seen in 3.6% of cases (Michels IX). Michels’ IX variant requires a twisting in anastomosis’ order since the artery has be sutured before the portal vein because of its deeper location posterior to the vein. An accessory left hepatic artery originating from the left gastric artery was found (Michels V). This accessory artery provides an additional source of arterial blood to the left hepatic lobe and may be sutured without compromising the arterial supply to the left hepatic lobe.
No cases of type IV, VI, VII, VIII or X abnormalities were found in our study, whereas the literature reports a mean prevalence of 0.6 ± 0.4, 3.1 ± 2.2, 0.4 ± 0.4, 0.4 ± 0.7 and 0.1 ± 0.2%, respectively [3].
On the other hand, Michels’ classification is only based on hepatic arteries variants. For this reason, we recognized 46 anatomical variants not classified on the basis of Michels’ classifications. However, our incidence of these variants did not differ from the literature may be because the visualization of this large vessels in MDCT is similar to conventional angiography or anatomical dissection [3].
A potential limitation of our study should be that it has been performed on a 4-detector row CT with the actual tendency to use higher detector CT. However, until now, a 4-detector row CT is the standard in many departments. Furthermore, our results highlight that good quality 3D rendered and MIP images may be acquired even with a 4-detector configuration scanner avoiding diagnostic DSA.
Conclusion
Understanding of celiac trunk anatomy as well as knowledge of the type of anatomical variants and their subtypes, detected in our experience in quite 28% of patients, is fundamental for a correct pre-operative vascular planning in surgical or radiological abdominal procedures. Multidetector-row CT (MDCT) provides high-quality 3D-reconstructed images and allows non-invasive assessment of normal anatomy and anatomic variants of celiac trunk.
References
Burger IM, Murphy KJ, Jordan LC et al (2006) Safety of cerebral digital subtraction angiography in children: complication rate analysis in 241 consecutive diagnostic angiograms. Stroke 37:2535–2539
Coskun M, Kayahan E, Ozbek O et al (2005) Imaging of hepatic arterial anatomy for depicting vascular variations in living related liver transplant donor candidates with multidetector computed tomography: comparison with conventional angiography. Transplant Proc 37:1070–1073
Ferrari R, De Cecco CN, Laghi A et al (2007) Anatomical variations of the coeliac trunk and the mesenteric arteries evaluated with 64-row CT angiography. Radiol Med 112:988–998
Hyare H, Desigan S, Nicholl H et al (2006) Multi-section CT angiography compared with digital subtraction angiography in diagnosing major arterial hemorrhage in inflammatory pancreatic disease. Eur J Radiol 59:295–300
Koops A, Wojciechowski B, Broering DC et al (2004) Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies. Surg Radiol Anat 26:239–244
Losanoff JE, Millis JM, Harland RC et al (2007) Hepato-spleno-mesenteric trunk. J Am Coll Surg 204:511
Matsuki M, Tanikake M, Kani H et al (2006) Dual-phase 3D CT angiography during a single breath-hold using 16-MDCT: assessment of vascular anatomy before laparoscopic gastrectomy. AJR Am J Roentgenol 186:1079–1085
Michels NA (1995) Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. Lippincott, Philadelphia, pp 139–143
Piquand G (1910) Recherches sur l’anatomie du tronc coeliaque et des ses branches. Bibliogr Anat 19:159–201
Prokop M (2000) Multislice CT angiography. Eur J Radiol 36:86–96
Redman HC, Reuter SR (1969) Angiographic demonstration of surgically important vascular variations. Surg Gynecol Obstet 129:33–39
Rubin GD, Shiau MC, Schmidt AJ et al (1999) Computed tomographic angiography: historical perspective and new state-of-art using multi-detector-row helical computed tomography. J Comput Assist Tomogr 23:S83–S90
Rubin GD (2000) Data explosion: the challenge of multidetector row CT. Eur Radiol 36:74–80
Rydberg J, Buckwalter KA, Caldemeyer KS et al (2000) Multisection CT: scanning techniques and clinical applications. Radiographics 20:1787–1806
Sahani D, Saini S, Pena C et al (2002) Using multidetector CT for preoperative vascular evaluation of liver neoplasms: technique and results. AJR Am J Roentgenol 179:53–59
Sone M, Kato K, Hirose A et al (2007) Impact of multislice CT angiography on planning of radiological catheter placement for hepatic arterial infusion chemotherapy. Cardiovasc Intervent Radiol 31(1):91–97 (Epub ahead of print)
Tandler J (1904) Über die varietäten der arteria coeliaca und deren entwickelung. Anat Hft 25:473–500
Willmann JK, Baumert B, Schertler T et al (2005) Aortoiliac and lower extremity arteries assessed with 16-detector row CT angiography: prospective comparison with digital subtraction angiography. Radiology 236:1083–1093
Winston CB, Lee NA, Jarnagin WR et al (2007) CT angiography for delineation of celiac and superior mesenteric artery variants in patients undergoing hepatobiliary and pancreatic surgery. AJR Am J Roentgenol 189:W13–W19
Winter T III, Freeny P, Nghiem H et al (1995) Hepatic arterial anatomy in transplantation candidates: evaluation with three-dimensional CT arteriography. Radiology 195:363–370
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iezzi, R., Cotroneo, A.R., Giancristofaro, D. et al. Multidetector-row CT angiographic imaging of the celiac trunk: anatomy and normal variants. Surg Radiol Anat 30, 303–310 (2008). https://doi.org/10.1007/s00276-008-0324-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-008-0324-7