Abstract
The esogastric anastomotic fistula,occurring after the replacement of esophagus by the stomach, is a post-operative complication always feared and awaited. Apart from other causes, there exist the anatomical dispositions notably the vascular and technical factors that stress this potential risk despite certain advantages of esophagogastroplasty. The goal of our study was to study the arterial distribution of the gastric transplants in order to identify the better modalities of their making. We used 39 stomachs taken from fresh cadavers of autochtone subjects. After a modeling treatment using three different techniques, they were subjected to a radiographic opacification of the right gastro-epiploic artery with sulphate of barium follow by an x-rays in incidence full-face (25 kv, 10 mAS). It was a matter of 15 entire stomachs (E.E.) with denudation of the small curvature, of 12 wide gastric tubes (W.T.) prepared according to the Akiyama technique modified and of 12 narrow tubes (N.T.) tubulized according to the Marmuse method. We studied the anastomotic type of the gastro-epiploic arterial circle according to the classification of Koskas, the collateral branches of the arterial circles of the gastric curvatures, the antral and corporeal anastomosis of these circles and the distribution anastomotic at the level of the summit of the anastomotic. Only 28 pieces (15 E.E., 8 W.T. and 5 N.T.) were able to be the object of a complete angiographic exploitation. The anastomosis of the arterial circle was type I in 64.1% of the cases, type II in 15.4% of the cases, type III in 15.4% of the cases and type IV in 5.1% of the cases. The average number of collateral branches originating from gastro-epiploic arterial circle was respectively 24, 17 and 22 for the E.E., the W.T. and the N.T. Only the two first ones presented collateral branches being borne of the small curvature circle. Fifty per cent of the N.T. did not possess any antral or corporeal anastomosis between the two arterial circles; some of them were even for a quarter of the W.T. In the case of gastric tubulization there existed an irrigation defect of the summit of the plasty for a third of the N.T. and a quarter of the W.T., despite a constant intramural bridge anastomosis between the two gastro-epiploic arteries. The usage of the entire stomach must be recommended for gastric oesophagoplasty; but when the operative indications require a resection of the small curvature it is preferable to use a wide gastric tube whose diameter respects the two left third of the initial width of the organ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The correlation between the occurrence of an oesogastric anastomotic fistula and the vascular deficit of a gastroplasty following the replacement of the esophagus has been established [1, 11]. The identification of the gastric territories that are little or not at all vascularized at the summit of the neo-esophagus is all the more important since the carrying out of an anastomosis on the neck does not always result in an arch-shaped recut of the cranial pole of the transplant as certain authors recommend [10]. The aim of our work was to study the cadavers for the distribution of the opacified gastric arterial network following the surgical confection of three types of gastric oesophagoplasty (OPG).
Materials and methods
We used 39 stomachs from recent melano-dermic adult cadavers with no pathologic or thoraco-abdominal surgical antecedents and free from traumatic lesions on the supra-mesocolic level. Following the monobloc removal of the organs at the supra-mesocolic level all of the viscera including the abdominal esophagus, the stomach and the first duodenum, were isolated by gastrolysis that took into consideration the gastro-omental vascular circle in accordance with the surgical procedure. The anastomotic mode between the right and left gastro-omental arteries was identified in accordance with the Koskas classification [12] (Fig. 1). This gastrolysis was completed with an intra-hilar splenectomy in order to take the short vessels into consideration. The organs were divided into three groups, the last two of which were subjected to gastric resection and manual edge-to-edge suture:
-
in an initial group composed of 15 whole stomachs, the small curve was stripped of the left gastric pedicle as in curettage.
-
in the second group composed of 12 organs, the gastroplasty was confectioned according to Akiyama’s method [2], that is to say, before the edge-to-edge gastrorraphy suture,it had a linear and oblique resection on the upper part and from right to left of the small curve to the summit of the fundus. The only difference with regard to the technique described by Akiyama [2] was that the resection started not under the first three collateral branches of the left gastric artery, but at the level of the fifth collateral branch (Fig. 2a, b) as recommended by Triboulet [17].
-
in a third group composed of 12 organs, there was a gastric tubulization along the great curve in accordance with Marmuse [15] that consisted in a wide resection of the right 2/3 of the stomach (Fig. 3a, b). This started at the bottom, 2 cm above the upper edge of the pylorus and ran along the great curve at a distance set at 4 cm, with the resection terminating at the top on the summit of the fundus.
For the last two groups, the tranches of gastric section were sutured in one plane by manual edge-to-edge suturing from bottom to top using a 3/0 polyglactine reabsorbable woven thread. The procedure in the laboratory was completed by a check for water-tightness of the gastric suture using Toluidine blue tinted water.
Classification by Koskas [12] of the anastomotic mode of the arterial gastro- omental circle of the great gastric curve. aged, a. right gastro-epiploic. ageg, a. left gastro-epiploic
Gastric oesophagoplasty using Marmuse’s technique [15], a gastrotomy and narrow tubulization, b narrow gastric tube
Thirty-nine of the 45 organs were subjected to arterial opacification; only 15 of the 21 whole stomachs were used. Each opacification of the right gastro-epiploic artery was carried out by the gentle manual injection of a solution of dilute barium sulphate (Micropaque®) using a 50 cc syringe.
Initially the progress of the radio-opaque product was monitored and checked by radioscopy.
Subsequently, when the contrasting product had ceased to progress, one or more X-rays were taken at frontal incidence; the constants used were 25 kV and 10 mA/s.
The X-rays were than labeled and subjected to interpretation. For each group, we studied the following parameters:
-
the type of anastomotic arcade of the gastro-epiploic arterial circle.
-
the number of collateral arterial branches that arise from the arterial circle of the great curve.
-
the existence and number of antral and/or corporeal anastomosis of significant caliber between the arterial circles of the two curves.
-
the number of visible arterial branches that irrigated the summit of the gastroplasties that were well vascularized.
-
the number of organs for which there was either satisfactory arterial distribution or significant devascularization of the summit of the plasty.
Results
Out of the 39 stomachs before gastroplasty, the macroscopic analysis of the anastomotic mode of the gastro-epiploic arterial circle according to the classification by Koskas [12] identified (Table 1): 23 type-I (58.9%), 9 type-II (23.07%), 5 type-III (12.82%) and 2 type-IV (5.12%).
An arteriographic study according to the same classification showed the following breakdown (Table 1):
-
type
I: 25 cases (64.1 %) including eight whole stomachs, eight wide tubes and nine narrow tubes.
-
type
II: six cases (15.4 %) including two whole stomachs, one wide tube and three narrow tubes.
-
type
III: six cases (15.4 %) including four whole stomachs (Fig. 4) and two wide tubes.
-
type
IV: two cases (5.1 %) including one whole stomach and one wide tube.
Furthermore, the angiographic study of the type I anastomosis in our series shows two models whose frequencies are similar: a type Ia, with a full canal anastomosis without modification in the size of the right and left gastro-epiploic arteries which are continuous from one to the other, constituting a true arterial arcade that runs along the great curve, and a type Ib, whose anastomosis is produced by the terminal branches of the right and left gastro-epiploic arteries which are smaller than that of the trunk.
The number of collateral branches that arise from the great curve varied and was often determined by the right gastro-epiploic artery, which provided the majority of them (Table 2). They numbered 24 per whole stomach for the gastroplasties, 17 for the wide tubes and 22 for the narrow tubes. For the latter, an average number of 12 gastro-epiploic branches crossed the entire width of the tube. In the vast majority of cases they were single at their origin but were able to divide rapidly into two branches, which sometimes crossed before reaching the great gastric curve. Occasionally, the emergence of 2 separate branches at the same level arising from different sides of the right gastro-epiploic artery was noted; each of them clearly progressed towards a gastric surface. The branches of the right gastro-epiploic artery were distinguished by their different length and the orientation of their progress depending on their original level. At the pyloro-antral level and in the initial part of the sub-antral progress of the right gastro-epiploic artery, there were many collateral branches close together with an ascendant vertical path whose extra-mural portion was short. As the end of the right gastro-epiploic artery was approached, its collateral branches were longer, for the arterial arcade gradually diverged from the great curve while they became less numerous and more spaced with an oblique path that went upward and to the right.
With regard to the antral and/or corporeal anastomosis that were clearly identifiable for the whole stomachs (Fig. 5), their number varied from three to nine with an average of five anastomosis, of which two were antral and three corporeal. For the wide tubes, the average number of these anastomosis was also five. These anastomosis did not exist in the narrow tubes.
The arterial branches that vascularized the upper part of the plasty were short vessels for the whole stomach; their number varied from one to five with an average of three short vessels. For the gastroplasties per tube, the average number of branches that evoked short vessels was three, both for the wide tubes and the narrow tubes.
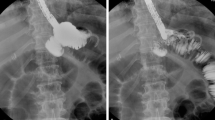
With regard to the vascularization of the summit of the gastric tubes, whether the plasties were wide or narrow, we noted the inclusion of an average number of three narrow arteries, but only 75 % of the wide tubes and 60 % of the narrow tubes (Fig. 6) showed elective vascularization of their summit. In the majority of cases, the latter resulted from the epiploic branches, which arose from the adjacent omentum (Fig. 7).
The topography of the territories that showed a lack of arterial vascularization varied. For seven of the whole stomachs, it was the left half of the fundus where no branch was opacified. On the other hand, its right part was copiously irrigated in particular by the cardio-tuberositary branches of the left gastric artery. For the tubular plasties, arterial devascularization relating to the upper part was significant for two wide tubes and two narrow tubes (Fig. 8) including one that had no anastomosis on the gastro-epiploic circle of the great curve. The level of gastroplasty concerned by this devascularization varied, but was significant for all four tubes. The density of the arterial network at the summit of the transplant was satisfactory for five wide tubes and three narrow tubes. Close observation of the radiographic images showed that there was still an intra-mural bridge along the great curve (Fig. 9) between the right and left gastro-epiploic arteries.
Discussion
This work was inspired by anatomical [5] and radio-anatomical works [1, 10–12, 14, 20], which attempt to pinpoint the anatomic parameters and in particular the vascular parameters to be taken into account when a gastric plasty is being made as part of the replacement of the esophagus by the stomach. Although this work used fresh organs, it does not have the merit of having been carried out in situ, like the work of Koskas [12]. Nevertheless the stages of gastrolysis and the confection of various models of gastric transplants reproduce the technical surgical conditions both from the point of view of the equipment (instruments and thread) and methods (gastrolysis, gastrectomy and manual sutures) that we use in hospital practice. This contributes to an evaluation of the technical faults that cause devascularization following the subsequent opacification of the arterial network. With regard to arterial opacification, the ideal is the Khoury-Hélou method [11], which is carried out in vivo and on patients with an anastomotic fistula. Furthermore, the relevance of corrosion preferred by Lieberman-Meffert [14] lies in taking into account of the tri-dimension and also the opportunity of making measurements, which cannot be envisaged with X-rays.
With regard to the anastomosis of the gastro-epiploic arterial circle of the great curve, a comparison of arteriographic data with the macroscopic results shows a divergence in the range of frequency of the various Koskas types [12]. It appears that the adiposity of the gastrocolic ligament in which the gastro-epiploic arterial arcade runs is a source of error during macroscopic determination of an anastomotic type. These established facts allow us to state that the frequency of type II whose rate decreases from 22.2 to 15.4% is overestimated and that certain anastomosis of types I, III and IV are incorrectly identified and mistakenly considered to be the absence of anastomosis. This is also true for some studies on corrosion [7, 14] which do not individualize types III and IV or which describe some gastro-epiploic anastomosis as “mediocre” in 27.5% of cases without specifying the type. The arteriographic study by Yamato [20] defines a classification that is partly modified and specifies the frequencies for 137 stomachs. Thus it is that type I (34.4%) that produces a direct extra-mural anastomosis whereas type II (15.3 %) is an absence of direct extra-mural anastomosis with the presence of a free interval that is sometimes “bridged” by a small intra-mural vessel. As for type III (44.5 %), this corresponds to an absence of anastomosis with a long free interval while type IV (5.8%) is an absence of direct anastomosis but with the presence of an artery along the free edge of the great omentum that allows an extra-mural anastomosis. Should there be an absence of anastomosis (type II), we observe, as Koskas describes [12], two gastro-epiploic networks separated by a distance that varies in length. In type III the extra-mural anastomotic branch is small, it describes a curve whose concavity is directed toward the great curve and shows either no or very few collateral branches intended for the stomach. As for the type II there is also an intra-mural bridge here. Apart from the specificity of happening at more than 4 cm away from the great curve, the two cases of type IV anastomosis that we observe produce significant descending collateral branches intended to vascularize the great omentum.
More than the anatomic model of type I anastomosis that we observe, it is their functional character that is important and under normal haemodynamic conditions it may be supposed that an anastomosis that is visible radiologically, whatever its type, is permeable. If a comparison is made between Yamato’s classification [20] and the classification we are borrowing from Koskas [12] we note that the definitions of types I and IV of these two classifications can be super-imposed but that only type IV is concordant with regard to frequency. Yamato’s type III [20] appears to correspond to Koskas’ type II [12] with Yamato having a rate of 44.5% which is strangely high when it is known that arteriography enables us to decrease the number of absences of anastomosis. For type I Hannoun [9] indicates an arteriographic rate of 23.5%, which contradicts our’s and Koskas’studies [12] but he does not specify whether this figure covers full canal anastomosis only. The notion of intra-mural communication between the two gastro-epiploic networks is also reported by Levasseur [13] who highlights its functional interest, as well as by Dei Paoli [6] who like us, observed it on all of his gastric arteriographs.
With an average number of 24 collateral branches for gastroplasties per whole stomach, we observed more branches than Levasseur [13] whose maximum number is 18. Vandamme [19], for his part, highlights the characteristic path of these collaterals in the antro-pyloric portion of the right gastro-epiploic artery while Diop [7] does this for his terminal portion. El Eishi [8], dealing with 60 cases mentions only 4 to 5 collateral branches coming from the left gastro-epiploic artery. These branches contract anastomosis with the collateral branches of the right gastro-epiploic. We find this description again for all of the whole stomachs with the particular characteristic also highlighted by El Eishi [8] that the anastomosis between the left gastro-epiploic branches intended for the body of the stomach and the short arteries of the stomach are slender. For the gastric tubes, the vascularization of the upper part appears to be conditioned by the existence or not of omental arterial branches coming from the remains of the gastro-splenic omentum. This is verified on all the arteriographs of plasties by tube whatever be their width.
With regard to anastomosis between the arterial circles of the two curves, our results confirm the major considerations of Agossou-Voyémé [1] who states that the line of anastomosis between the arterial circles of the small and great curves is situated at the join of the right 1/3rd and left 2/3rds of the width of the stomach and who recommends the confection of a gastric tube whose width is greater than or equal to the left 2/3rds of the initial width of the stomach used. Furthermore, it is noted that there is no difference between gastroplasties by wide tube and those produced using the whole stomach with regard to the average number of highly visible anastomosis, which is five. This is explained by the fact that for wide tubes the line of incision of the gastrectomy always passes to the right of the line of anastomosis. This line is incidentally clearly identified in Liebermann-Meffert’s study by corrosion of gastric plasties [14], which observes that it runs along the great curve at a distance of 4–5 cm. Thus the epiploic branches that emanate from the arterial circles of the two curves constitute the principal vectors of the vascularization of the transplant and are fortunately supplanted by the inter-parietal anastomotic networks, in particular the sub-mucous network [16].
The average number of short vessels that we are able to identify on gastroplasties per whole stomach is in line with that observed by other authors [8,13]. In view of the fact that these short arteries may arise as easily from the left gastro-epiploic artery as from a superior polar splenic artery or the trunk of the splenic artery, it is certain that gastrolysis does not spare all the short arteries and that, in spite of a splenectomy at the base of the hilum, these are essentially the arteries of gastro-epiploic origin that are opacified. For short arteries of this origin, it is noted that their path is often horizontal to the right or slightly upward and to the right and that their length varies and is sometimes longer than their name implies, as Vandamme [19] points out. In the majority of cases they terminate in an initial bifurcation each of whose branches produces secondary branches, which anastomose in particular with the terminal branches of the first collateral branches of the left gastric artery. These anastomosis are produced at the upper third of the body of the stomach. In our radiographs, the short vessels intended for the fundus are rarely opacified since they are often sectioned during gastrolysis, but it is shown [15, 18] that these vessels irrigate the upper half of the great curve and in particular the left part of the fundus. Thus Collard [3] highlights the importance of producing wide gastric tubes when the use of the fundus is envisaged, which is the case for oeso-gastric anastomosis at the neck since it is necessary to preserve the maximum length of stomach because of the path to be covered.
In spite of the attention given to gastrolysis in our study, there are 25% wide tubes and 40% narrow tubes whose summit is little or not at all vascularized. In favorable cases, this summit is seen to be most often irrigated by epiploic branches from the left gastro-epiploic artery, whose path is oblique below and to the right or even vertical and descending when the gastro-epiploic omentum that enters the left edge of the tube reaches its summit. With regard to the territory of the fundus that is not vascularized, it may be allowed that for the seven gastroplasties per whole stomach involved, it is a question of the unintentional section of short vessels during gastrolysis when note is taken of the wealth of gastric anastomosis that have enabled us to obtain total irrigation of the other organs with the single injection of the right gastro-epiploic artery. Where there is devascularization of the gastric tubes, this always occurs at a significant height that reflects the iatrogenic interruption of the feeder arteries to the upper part of the transplant. Vandamme [19] recalls that a significant part of the great curve is not irrigated by the left gastro-epiploic artery but by the short vessels and Collard [4] makes it clear that the length of the arterial arcade is just 47–80% of that of the great curve. For his part, Liebermann-Meffert [14] summarizes the sources of vascularization of the gastric tubes with reference to their length, estimating that approximately 60% of their length is irrigated by the right gastro-epiploic artery, that 20% benefit from the collateral branches of the circle of the two gastro-epiploic arteries and that the cranial 20% of the length of these tubes are in the main irrigated by the dense sub-mucous network of small arteries.
Conclusion
The angiographic study of these three methods of OPG. reveal that there is always an intra-parietal gastric anastomosis, however small, between the right and left gastro-epiploic arteries and that the use of the whole stomach should be preferred. Nevertheless, when the surgical procedure for the oesophagectomy requires the resection of the small curve, a gastric tube whose width is at least 5 cm should be preferred in view of the fact that the minuteness of the gastrolysis in respect of the origin of the left gastro-epiploic artery and the short vessels is capital for the preservation of all of the vascular branches at the summit of the transplant.
References
Agossou-Voyémé AK, Hureau J, Germain MA (1990) Etude des problèmes vasculaires dans les o.p.g. après oesophagectomie ou pharyngo-laryngectomie circulaire. J Chir 127:141–149
Akiyama H, Miyazono H, Tsurumaru M, Hashimoto C, Kawamura T (1978) Use of the stomach as an esophageal substitute. Ann Surg 188:606–610
Collard JM, Otte JB, Kestens PJ (1990) Plastie oesophagienne utilisant l’estomac entier avec dénudation de la petite courbure. Lyon Chir 86:173–174
Collard JM, Tinton N, Malaise J, Romagnoli R, Otte JB, Kestens PJ (1995) Oesophageal replacement: gastric tube or whole stomach? Ann Thorac Surg 60:261–267
Cordier G, Debray C, Thomas J, Cabrol C (1955) Données récentes sur la vascularisation de la paroi gastrique. Conséquences pathologiques. Ann Chir 9:538–544
Dei Paoli M, Albertino B, Spalluto F, Andorno E, Bertero D, Gasparri G, Casalegno P, Ferrarotti G, Oliaro A (1990) Gastric angiography: data for oesophagogastroplasty. Panminerva Med 32:61–64
Diop M (1993) La vascularisation de l’estomac: données nouvelles sur la distribution des artères gastriques (à propos de 65 injection-corrosions) Thèse Médecine, Dakar, No. 3
El Eishi H, Ayoub JF, El Khalek M (1973) The arterial supply of the human stomach. Acta Anat 86:565–580
Hannoun L, Le Breton C, Bors V, Helenon C, Bigot JM, Parc R (1984) Radiological anatomy of the right gastroepiploic artery. Anat Clin 5:265–271
Khosrovani C, Le Neel JC, Kohen N, Guiberteau B, Vayre P (1994) Technique de gastroplastie oesophagienne isopéristaltique. Etude comparative de la vascularisation artérielle in vitro. J Chir 131:10–16
Khoury-Hélou A, Nonent M, Vandenbrouke F, Topart P, Lozac’h P (2001) Le déficit vasculaire est la cause majeure des fistules en chirurgie oesophagienne. Ann Chir 126:857–862
Koskas F, Gayet B (1985) Anatomical study of retrosternal gastric esophagoplasties. Anat Clin 7:237–256
Levasseur JC, Couinaud C (1968) Etude de la distribution des artères gastriques. J Chir 95:57–78
Liebermann-Meffert D, Meier R, Siewert J (1992) Vascular anatomy of the gastric tube use for esophageal reconstruction. Ann Thorac Surg 54:1110–1115
Marmuse JP (1988) Technique de l’oesophagectomie trans-hiatale. J Chir 125:585–592
Thomas D, Langford R, Russel R, Le Quesne L (1979) The anatomical basis for gastric mobilisation in total oesophagectomy. Br J Surg 66:230–233
Triboulet JP (1999) Intervention de Akiyama pour cancer de l’œsophage haut situé . J Chir 136:23–28
Vandamme JP, Bonte J (1986) Systématisation of the arteries in the splenic hilus. Acta Anat 125:217–224
Vandamme JP, Bonte J (1988) The blood supply of the stomach. Acta Anat 131:89–96
Yamato T, Hamonaka Y, Hirata S, Sakai K (1979) Esophagoplasty with an autogenous tubed gastric flap. Ann J Surg 137:197–202
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ndoye, JM., Dia, A., Ndiaye, A. et al. Arteriography of three models of gastric oesophagoplasty: the whole stomach, a wide gastric tube and a narrow gastric tube. Surg Radiol Anat 28, 429–437 (2006). https://doi.org/10.1007/s00276-006-0129-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-006-0129-5