Abstract
Purpose
To evaluate the effect of being under time pressure on procedural performance using hand motion analysis.
Materials and Methods
Eight radiology trainees performed central venous access on a phantom while recording video and hand motion data using an electromagnetic motion tracker. Each trainee performed the procedure six times: the first three trials without any prompts (control), while for the next three, they were asked to perform the task as fast as possible (time pressure). Validated hand motion metrics were analyzed, and two blinded and independent evaluators rated procedural performance using a previously validated task-specific global rating scale (GRS). Motion/time ratios and linear mixed-effect methods were used to control for time, and constants for both strategies were compared.
Results
Hand motion analysis showed that trainees completed the simulated procedure faster under time pressure (46 ± 18 s vs. 56 ± 27 s, p = 0.008) than during the control strategy. However, when controlling for time, trainees moved their hands 79 more centimeters (p = 0.04), made 15 more translational movements (p = 0.003) and 18 more rotational movements (p = 0.01) when under time pressure compared to at their own pace.
Conclusion
Although trainees could perform the procedure faster under time pressure, there was a deterioration in hand motion economy and smoothness. This suggests that hand motion metrics offer a more comprehensive assessment of technical performance than time alone.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hand motion analysis is an evolving method of evaluating technical skill in medical procedures [1,2,3]. Prior studies have supported the presumption that expert physicians are more efficient and steady with their hands when performing a given procedure than trainees [2, 4,5,6]. This was done through the study of motion metrics such as the total distance the hands traveled to complete a task (path length), the number of discrete linear movements of the hand (translational movements), the number of discrete rotations of the hand (rotational movements) and the time to complete the procedure. In clinical practice, improved procedural speed can translate to reducing the operating room time, thereby decreasing the associated costs and improving overall health economics. Furthermore, longer procedure time has been associated with higher complication rates and mortality in patients undergoing bariatric surgery [7]. Motion economy has been highly correlated with the level of expertise and could be associated with clinical outcomes.
Given that experts are expected to perform a manual task faster, the question arises as to whether time alone can be used as a quantitative measure of technical proficiency in lieu of other calculated metrics of economy and smoothness of motion.
While it seems evident that a procedure can be performed quickly but in an inefficient fashion, this has not been clearly proven. This study was designed to determine if the relationship between time and hand motion metrics is always fixed, by asking trainees to perform a common interventional task (ultrasound-guided central venous access) both at their own pace and under time pressure.
This has the secondary benefit of evaluating the effects of time pressure on trainee hand motion. This common clinical scenario may lend insight into the biometric technical performance of trainees who are under real or perceived stress. Understanding these effects could tailor educational programming to match real-world expectations with respect to biometric performance in an in vivo procedure, which has not previously been a specific focus of training.
Methods and Material
Participants and Settings
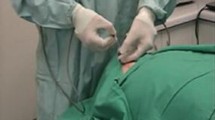
With IRB (Institutional Review Board) approval, eight radiology trainees (PGY3-PGY6) were voluntarily recruited to perform a simulated central venous access on a standardized manikin (SimuLab Central Line System, Seattle, Washington), while their hand motion was recorded using an electromagnetic motion tracker (Polhemus Liberty, Colchester, Vermont). All participants had previous experience with ultrasound-guided central venous line placement (ranging from 11 to 50 procedures for juniors and 51–100 for senior trainees). Motion sensors (Teardrop Mini, Polhemus) were attached to the dorsum of the needle hand and the base of the ultrasound probe (Fig. 1). All the participants performed the procedure six times (trials). They were not told the purpose of the study other than to evaluate their hand motion in simulated central venous catheter placement. They were asked to perform the first three trials at their own pace without any prompt (control). Then, for the next three trials, they were asked to perform the same task as fast as possible (time pressure), with live feedback on the time taken displayed on the screen of a digital stopwatch for the entire trial.
All the trials were also video-recorded. Two experienced interventional radiology attendings (7–10 years of IR practice, with previous experience in performance rating) evaluated these videos and independently scored the subject’s performance using an adapted global rating scale (attached) [8]. Raters were blinded to the identity of the participants, and this was achieved by asking all the participants to wear surgical gloves and positioning the camera such that only the subject’s hands were visible (Fig. 1).
Simulated Central Venous Access
For all the trials, a standardized manikin (SimuLab Corporation, Seattle, Washington) and a Butterfly iQ + ultrasound probe (Butterfly Network, Guilford, Connecticut) were used. The initial setup was also standardized by placing the guide wire on top of the manikin, the needle and dilator on the dominant hand side and the probe on the nondominant hand side.
Starting from a standardized position with hands on the manikin, participants were tasked with visualizing the carotid artery and internal jugular vein using the ultrasound probe. Subsequently, they were to use a syringe needle to access the jugular vein under ultrasound guidance, aspirating as they advanced. Upon successfully aspirating “blood” in the syringe, they were to detach the syringe from the needle and thread the guidewire through it. The participants were then asked to confirm its appropriate position within the jugular vein using the ultrasound probe, checking both the longitudinal and transverse axes. Following this, they were to thread a dilator through the wire until reaching the “skin” of the manikin, thereby marking the trial’s endpoint.
Motion Data Collection and Analysis
An electromagnetic motion tracking system (Polhemus Liberty, Colchester, Vermont) was used for hand motion recording with a sampling rate of 240 Hz. Raw data were exported to R 4.3.1 (R foundation for statistical computing, Vienna, Austria) and an institution-developed script was used to calculate the validated hand motion metrics as previously described [3].
Global Rating Scales
A five-item global rating scale for the assessment of central line placement was adapted from two validated global rating scales: Direct Observation of Procedural Skills (DOPS; The Foundation Program 2009) [9] and the Objective Structured Assessment of Technical Skills (OSATS; Reznick et al. 1997) [10]. Items on the original scales not applicable to simulator examination were removed [8]. All of the items were scored from 1 to 5 and a global performance score was calculated at the end.
Statistical Analysis
Data analysis was performed in R 4.3.1. The Shapiro–Wilk tests showed that all metrics were non-normally distributed so nonparametric techniques were used.
Linear mixed-effect methods were used (nlme package in R) to individually model the three metrics (path length, translational movements and rotational movements) against the time taken for the procedure (fixed effect), with the participants acting as random effects.
Controlling for time between the time pressure and control condition was performed in two different ways to ensure uniformity of the findings: (1) the ratios of motion metrics over time were calculated and compared; (2) the linear mixed-effects model was used (as done in a previous surgical study [11]), to predict the motion metric values at 60 s in both the time pressure and control conditions. This model was used to prevent pseudo-replication due to multiple observations on the same subjects.
Inter-rater reliability was assessed using the Cohen’s Kappa coefficient. Mann–Whitney U tests were used for independent samples and Wilcoxon signed-rank for paired sample. Correlation analysis was performed using Spearman’s rank correlation.
Results
Motion data were recorded for 48 trials from the 8 participants. One of the trials was excluded from the analysis because the participant did not fully execute the standardized steps (unable to secure access in the internal jugular vein). Therefore, a total of 47 trials were included in the analysis.
Time-movement correlation plots showed a high correlation for both strategies using the regression model (Fig. 2).
Analysis of motion data showed that when placing the line under time pressure, the trainees completed the procedure faster (48.9 ± 19 s vs. 68.9 ± 19.7 s, p < 0.001) (Table 1). Comparing the motion metrics between time pressure and control using the first method (the ratios of motion metrics per unit time), there was a significant increase in the number of translational and rotational movements per second (p < 0.001) when performing the procedure under time pressure (Table 2).
When using the second method to control for time (the linear mixed-effects model), trainees move their hands 79 more centimeters, use 15 more translational movements and 18 more rotational movements at 60 s when performing the procedure quickly (Table 3).
The inter-rater reliability of the GRS scores between evaluators had a Kappa coefficient of 0.12 (95% CI = − 0.08, 0.33) reflecting no agreement. Wilcoxon signed-rank tests showed no difference in GRS scores between time pressure and control performance for both raters (Rater-1; p = 0.29 and Rater-2; p = 0.11).
Spearman’s rank correlation coefficient between GRS and motion metrics showed a high negative correlation for rater-1 and negligible correlation for rater-2. Using the mean GRS, it showed low to moderate negative correlation (Table 4).
Discussion
Attending physicians perform procedures faster than trainees in addition to being more efficient with their movements, resulting in moving their hands a shorter distance with fewer total movements. This has been borne out in prior studies of hand motion analysis in medical specialties such as surgery [1, 12, 13] and, more recently, interventional radiology [3, 4]. While biomechanics can only be assessed using specialized equipment, the tight correlation between biomechanical expertise and decreased time to complete a task raises the question of whether using time alone is sufficient to determine proficiency. While there is a correlation, time alone is a blunt instrument. It is plausible to do a procedure in a fast but haphazard manner, making the distinction between time and motion relevant. This means that time alone, in theory, is not sufficient to determine technical proficiency.
Having trainees perform a simulated task routinely and then under time pressure allowed for analysis of the time-motion relationship in different circumstances. Performing the simulated procedure several times before applying time pressure has the potential to confound the results, allowing trainees to become more comfortable and proficient with the task through repetition and practice. Despite this advantage, trainees needed to move their hands a longer total distance and made more movements when controlling for procedure time. This means that biomechanically the trainees did worse when focusing on doing the procedure quickly. This helps quantitatively establish that while improved efficiency may typically correlate with time, they are distinct, and hand motion analysis provides a better picture of technical performance.
Separately, if hand motion analysis is to play a role in the future assessment of technical performance, its comparison to an observational standard assessment is useful. As was seen in the results, the agreement between two interventional radiology attending observers was poor. This speaks to the subjectivity of observation alone and reflects the real-world situation of trainees being evaluated “on the job” by the attendings who work with them. In this case, the observers were given a global rating scale that was adopted from an evaluation tool that was validated in surgery [8].
Aside from visual assessment being subjectively biased, the results could be due to having only two observers, the tool not being transferable to radiologic procedures, inadequate training of the observers on the rating questionnaire or the inherent insensitivity of observational assessment of fine motor skills compared to electromagnetic motion tracking equipment. Regardless, having several observers and specialized observer training to ensure uniformity of scoring represents a cost that should be weighed against the efficiency of developing hand motion metric standards that a computer and motion-tacking equipment can collect and process in an objective fashion.
While the task in this study was a simulated one, it bears mentioning that just asking the trainees to perform quickly and being aware of being assessed on speed resulted in a measurable decline in biomechanical performance. The decline in hand motion metrics may translate to any state of anxiety or pressure, such as being observed by an expert, the declining or critical nature of a patient’s health intraprocedurally or lack of patient cooperation with a procedure, among other factors. This could be an area of future study. Simulated practice may develop more comfort and result in a smaller degradation of performance under pressure in in vivo procedures. If so, this may help justify a simulation program in interventional radiology training curricula.
There are some limitations. This study only used one procedure with its own limited realism and translatability to other procedures or performance in real life. The time pressure was self-imposed, with each trainee applying varying levels of pressure to perform the procedure faster, which was not quantified with surveys or biometric data such as galvanic skin response or changes in vital signs. All participants performed the procedure faster under time pressure, indicating that some degree of urgency was experienced by every trainee. Additionally, the rating scales were performed on videos of the procedures rather than being present at the time they were performed, which may have allowed the observer to have different angles of the procedure. Lastly, it is hard to define the clinical benefit of better hand motion metrics—a procedure performed inefficiently could meet the same safety endpoints as one that was performed smoothly if the incidence of complications for the procedure is inherently low.
Conclusion
This study supports a limited amount of prior research, suggesting that time alone is not sufficient to determine technical proficiency and that hand motion metrics provide additional objective information about technical performance. The impact of time pressure on a trainee has the expected effect of degrading technical performance, as evinced by worsening hand motion metrics. Further research should focus on identifying the clinical impacts of different hand motion biomechanical profiles on patients’ outcomes and impact on overall health economics.
References
Chin KJ, Tse C, Chan V, Tan JS, Lupu CM, Hayter M. Hand motion analysis using the imperial college surgical assessment device: validation of a novel and objective performance measure in ultrasound-guided peripheral nerve blockade. Reg Anesth Pain Med. 2011;36:213–9. https://doi.org/10.1097/AAP.0b013e31820d4305.
Clinkard D, Holden M, Ungi T, Messenger D, Davison C, Fichtinger G, McGraw R. The development and validation of hand motion analysis to evaluate competency in central line catheterization. Acad Emerg Med. 2015;22:212–8. https://doi.org/10.1111/acem.12590.
Weinstein JL, Ali H, Sarwar A, Dadour JR, Brook OR, Mitchell JD, Matyal R, Palmer MR, MacLellan C, Ahmed M. evaluation of technical performance of ultrasound-guided procedures through hand motion analysis: an exploration of motion metrics. J Vasc Interv Radiol. 2023;34:1337–44. https://doi.org/10.1016/j.jvir.2023.05.015.
Weinstein J, El-Gabalawy F, Sarwar A, DeBacker SS, Faintuch S, Berkowitz S, Bulman J, Palmer M, Matyal R, Mahmood F, Ahmed M. Analysis of kinematic differences in hand motion between novice and experienced operators in interventional radiology: a pilot study. J Vasc Int Radiol. 2021;32(2):226–34.
Ghasemloonia A, Maddahi Y, Zareinia K, Lama S, Dort JC, Sutherland GR. Surgical skill assessment using motion quality and smoothness. J Surg Educ. 2017;74:295–305. https://doi.org/10.1016/j.jsurg.2016.10.006.
Smith SGT, Torkington J, Brown TJ, Taffinder NJ, Darzi A. Motion analysis: A tool for assessing laparoscopic dexterity in the performance of a laboratory-based laparoscopic cholecystectomy. Surg Endosc. 2002;16:640–5. https://doi.org/10.1007/s004640080081.
Birkmeyer JD, Finks JF, O’Reilly A, Oerline M, Carlin AM, Nunn AR, Dimick J, Banerjee M, Birkmeyer NJO. Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369:1434–42. https://doi.org/10.1056/NEJMsa1300625.
Ma IWY, Zalunardo N, Pachev G, Beran T, Brown M, Hatala R, McLaughlin K. Comparing the use of global rating scale with checklists for the assessment of central venous catheterization skills using simulation, Adv in Health. Sci Educ. 2012;17:457–70. https://doi.org/10.1007/s10459-011-9322-3.
SLE types, UK Foundation Programme (n.d.). https://foundationprogramme.nhs.uk/curriculum/supervised-learning-events/sle-types/ (accessed December 22, 2023)
Reznick R, Regehr G, MacRae H, Martin J, McCulloch W. Testing technical skill via an innovative “bench station” examination. Am J Surg. 1997;173:226–30. https://doi.org/10.1016/S0002-9610(97)89597-9.
Mackay S, Datta V, Mandalia M, Bassett P, Darzi A. Electromagnetic motion analysis in the assessment of surgical skill: relationship between time and movement: relationship between time and movement is not fixed for a standardized task. ANZ J Surg. 2002;72:632–4. https://doi.org/10.1046/j.1445-2197.2002.02511.x.
McGoldrick RB, Davis CR, Paro J, Hui K, Nguyen D, Lee GK. Motion analysis for microsurgical training: objective measures of dexterity, economy of movement, and ability. Plast Reconstr Surg. 2015;136:231e–40e. https://doi.org/10.1097/PRS.0000000000001469.
Datta V, Mackay S, Mandalia M, Darzi A. The use of electromagnetic motion tracking analysis to objectively measure open surgical skill in the laboratory-based model. J Am Coll Surg. 2001;193:479–85. https://doi.org/10.1016/S1072-7515(01)01041-9.
Acknowledgements
A similar version of this project was accepted as an abstract at the SIR 2024 meeting.
Funding
This study was funded by the Controlled Risk Insurance Company of Vermont (CRICO). The sponsor did not participate in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Metrouh, O., Ali, H., DeBacker, S.E.S. et al. The Effect of Time Pressure on Motion Economy and Smoothness of Interventional Radiology Trainee Performance in Simulated Central Venous Line Placement. Cardiovasc Intervent Radiol (2024). https://doi.org/10.1007/s00270-024-03831-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00270-024-03831-9