Abstract
Purpose
Optimal systolic blood pressure (SBP) management during endovascular treatment (EVT) for acute ischemic stroke remains a topic of debate. Though BP is associated with worse functional outcome, the relationship between BP and post-procedural intracranial hemorrhage (ICH) is less well-known. We aimed to investigate the association between BP during EVT and post-procedural ICH on dual-energy CT (DECT).
Methods
We included all patients who underwent EVT for an anterior circulation large vessel occlusion between 2010 and 2019, and received DECT < 3 h post-EVT. All BP measurements during the EVT procedure were used to calculate mean arterial pressure (MAPmean), mean SBP (SBPmean), and SBPmax-min (highest minus lowest). ICH was assessed using virtual post-procedural unenhanced DECT reconstructions and classified as intraparenchymal or extraparenchymal. Symptomatic ICH was scored according to the Heidelberg criteria. The association between different BP parameters and ICH was assessed using multivariable logistic regression.
Results
We included 478 patients. Seventy-six patients (16%) demonstrated ICH on DECT, of which 26 (34%) were intraparenchymal. Symptomatic intraparenchymal and extraparenchymal ICH occurred in 10 (38%) and 4 (8%) patients. SBPmax, SBPmean, and MAPmean were associated with intraparenchymal ICH with an adjusted odds ratio of 1.19 (95%CI, 1.02–1.39), 1.22 (95%CI, 1.03–1.46), and 1.40 (95%CI, 1.09–1.81) per 10 mmHg, while BP was not significantly associated with extraparenchymal ICH. BP did not differ between asymptomatic and symptomatic ICH.
Conclusion
Procedural BP is associated with intraparenchymal ICH on post-EVT DECT but not with extraparenchymal ICH. Future studies should evaluate whether individual procedural BP management reduces post-EVT ICH and improves clinical outcome.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the fact that endovascular treatment (EVT) for acute ischemic stroke is considered safe, the procedure carries a risk of intracranial hemorrhage (ICH) [1, 2]. This risk of ICH post-EVT can be attributed to mechanical lesions of the vessels that occur during EVT or reperfusion injury of brain tissue after recanalization [2, 3].
Previous studies have indicated that blood pressure (BP) may be associated with an increased risk of ICH post-EVT [2, 4]. However, these studies mainly focused on BP after, rather than during EVT. Furthermore, other studies which evaluated BP during EVT mainly assessed the relationship between decreases in BP and ICH, whereas research on the relationship between elevated BP during EVT and post-EVT ICH is sparse [5,6,7]. One study showed that BP at the time of recanalization is associated with ICH post-EVT. However, the presence of ICH was assessed on non-contrast CT between 24 and 36 h, and post-EVT elevated BP up until the non-contrast CT was not taken into account [4, 8, 9]. Moreover, assessment of ICH on non-contrast CT could lead to an overestimation of ICH due to the inability to reliably distinguish between blood and contrast [10]. An immediate post-EVT dual-energy CT (DECT) is able to overcome these limitations, as well as the ability to reliably distinguish between blood and contrast [11].
In this study, we aimed to evaluate the association between procedural BP and post-EVT ICH on DECT, in patients who underwent EVT for acute ischemic stroke.
Methods
Patient Selection
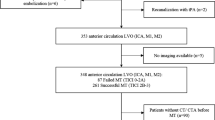
After gaining approval of the local ethics committee who waived the need for individual patient consent (METC-2020-1456), we screened all EVT records at the Maastricht University Medical Center from January 2010 up and including December 2019. Patients were considered eligible for inclusion if they had occlusion of the intracranial carotid artery (ICA), intracranial carotid artery terminus (ICA-T) or middle cerebral artery (M1/M2) and received DECT within 3 h post-EVT. Patients with pre-EVT ICH (e.g., chronic subdural hematoma or ICH due to head trauma) were excluded. Patients included in the MR CLEAN LATE trial were excluded, due to the blinded endpoint of this trial [12]. Likewise, patients receiving additional aspirin or heparin as part of MR CLEAN MED treatment allocation were excluded, since it increases the risk of ICH [13]. Patients receiving aspirin or heparin during EVT as part of regular care were not excluded.
Blood Pressure
BP measurements during EVT procedure were retrieved retrospectively from the anesthesia registration forms. As part of regular care, procedural BP generally was measured by using a noninvasive BP cuff or by using invasive BP measurements via an intra-arterial catheter and was generally recorded every three to five minutes. For our analysis, we did not apply a minimum number of measurements because this might introduce a bias by excluding patients with a very fast procedure time. In every patient, we calculated mean systolic BP (SBPmean) and mean diastolic BP (DBPmean). Mean arterial pressure (MAP) was calculated using the following formula: MAPmean = DBPmean + 1/3 (SBPmean − DBPmean) [14]. To describe BP variability, SBPmax–min was calculated subtracting the lowest SBP from the highest SBP, which has the advantage of being independent of SBPmean [15].
Anesthetic Management
During the EVT procedure, standard monitoring was applied including a 3- or 5-lead electrocardiogram, pulse oximetry, and BP monitoring. Anesthetic management consisted preferably of local anesthesia alone, but local anesthesia with procedural sedation and analgesia, or general anesthesia, could be applied up to the discretion of the responsible anesthesiologist. Generally, remifentanil and propofol were used for procedural sedation and analgesia or general anesthesia. Norepinephrine, phenylephrine, or ephedrine was used for inotropic and/or vasoactive treatment, while labetalol, clonidine, or urapidil was used for antihypertensive therapy. However, BP management during EVT was not protocolized. Therefore, hemodynamic management was also up to the discretion of the anesthesiologist. Hemodynamic interventions during EVT were retrospectively obtained from patients records, and details are presented in Supplemental Table 1.
Imaging Protocol
DECT was performed immediately, as soon as logistics allowed, post-EVT as part of regular care in our center and was performed on a second- or third-generation dual-source CT system (Somatom Definition Flash/Force, Siemens Healthcare, Forchheim, Germany). The dedicated dual-source protocol has been described elsewhere [16]. Postprocessing was performed using Syngo.via (VB40, Siemens Healthcare, Munich, Germany), which uses a three-material decomposition algorithm to differentiate between normal brain parenchyma, iodine concentration (by creating iodine overlay reconstructions), and ICH (by creating virtual post-procedural unenhanced reconstructions).
Imaging Assessment
The virtual post-procedural unenhanced reconstructions of DECT were assessed for ICH by a neuroradiologist with > 10 years of experience with DECT (A.P). ICH was scored according to the Heidelberg Criteria [17]. If a patient had both an intraparenchymal and extraparenchymal ICH, the ICH was scored as intraparenchymal, since we hypothesize that BP influences the risk of intraparenchymal ICH more than extraparenchymal ICH [18, 19]. All patients with ICH on DECT were screened for the occurrence of symptomatic ICH. Symptomatic ICH was scored according to the Heidelberg Bleeding Classification and was defined as an ICH with neurological deterioration toward admission National Institutes of Health Stroke Scale (NIHSS) of ≥ 4 points on the total NIHSS score or ≥ 2 points on any NIHSS category, or an ICH leading to surgical intervention [17]. Patients with ICH who were asymptomatic at the time of DECT, regardless of whether symptoms developed later on, were classified as asymptomatic.
Statistical Analysis
BP measurements were missing in 27% of the patients due to missing anesthesia registration forms. Data regarding clinical variables were gathered from prospective records and were overall only missing in 3%. Missing data were imputed using both single and multiple imputations by chained equations with the “mice” package (version 3.14.0) [20]. The single and multiple imputation models included the same relevant covariates. The number of imputed datasets needed to have consistent estimates of the standard error was based on the fraction of missing information (n = 23) [21].
Baseline characteristics were compared between patients without ICH and patients with intraparenchymal or extraparenchymal ICH using conventional statistics on the crude dataset.
Univariable and multivariable binary logistic regression was used to assess the association between each BP parameter and the occurrence of intraparenchymal or extraparenchymal ICH in the multiple imputed datasets. Patients with intraparenchymal ICH were excluded from the regression models analyzing BP and isolated extraparenchymal ICH, and vice versa. To avoid overfitting, the multivariable models were only adjusted for EVT duration and number of attempts during EVT, since this could be indicative for a more challenging procedure and a higher chance of complications [3, 22, 23]. However, based on univariable analysis and previous literature, four sensitivity analyses with four different pairs of adjustments were performed to investigate the robustness of the results. Additionally, an interaction effect of procedural BP with recanalization status was evaluated. Recanalization status was assessed using the expanded treatment in cerebral infarction (eTICI) score [24]. Successful recanalization was defined as eTICI ≥ 2B. All results are presented per 10 mmHg increase in BP.
In order to assess whether BP parameters differed between asymptomatic and symptomatic patients with either extraparenchymal or intraparenchymal ICH, Mann–Whitney U tests on a single imputed dataset were performed. As the time between EVT and DECT might be too short to develop a symptomatic ICH, a sensitivity analysis was performed in which patients with asymptomatic ICH at time of DECT were classified as symptomatic if neurological deterioration occurred within 24 h post-EVT. Lastly, a sensitivity analysis was performed excluding patients with missing BP measurements.
A P value < 0.05 was considered significant. Due to the exploratory nature of this study, no adjustments for multiple testing were applied. All statistical analysis were performed using R version 4.2.2.
Results
We included 478 patients in our study (Fig. 1). The median available number of procedural BP measurements per patient was 9 (IQR 6–15). Invasive BP measurements via an intra-arterial catheter only occurred in three patients. The mean of the procedural SBPmean, SBPmax, and MAPmean were 150 ± 26 mmHg, 173 ± 29 mmHg, and 101 ± 17 mmHg, respectively (Supplemental Fig. 1).
76/478 (16%) patients showed ICH on post-EVT DECT, of which 50 (66%) were classified as extraparenchymal (of which 44 subarachnoid ICH, Supplemental Table 2) and 26 (34%) as intraparenchymal. Baseline characteristics and procedural BP of patients without ICH, patients with intraparenchymal ICH, and patients with extraparenchymal ICH are shown in Table 1. Patients with an intraparenchymal ICH more often took antiplatelet medication pre-stroke (P = 0.006) and had a higher SBPmax, SBPmean and MAPmean (P = 0.02, P = 0.002, and P = 0.006, respectively) compared to patients without ICH (Table 1). Patients with an extraparenchymal ICH had a higher number of attempts during the procedure (P < 0.001), less often achieved successful recanalization (P = 0.04), more often had a perforation as complication during EVT (P < 0.001), had longer EVT duration (P = 0.03), and less often had general anesthesia (P = 0.004) compared to patients without ICH, but no significant differences in BP parameters. Overall, there was no significant difference in frequency of IVT administration in patients with intra- or extraparenchymal hemorrhage and patients without ICH.
Intraparenchymal and Extraparenchymal ICH
The results of multivariable regression analysis are presented in Table 2. Intraparenchymal ICH was associated with SBPmax, SBPmean, and MAPmean with adjusted odds ratios of 1.19 (95%CI 1.02–1.39), 1.22 (95%CI 1.03–1.46), and 1.40 (95%CI 1.09–1.81) per 10 mmHg increase, respectively. Extraparencymal ICH was not associated with procedural BP. Moreover, four additional models, adjusted for other significant confounders, are presented in Supplemental Tables 3–6 and did not yield different results. There was no significant interaction between BP and recanalization status on intraparenchymal or extraparenchymal ICH (Supplemental Table 7).
Asymptomatic and Symptomatic ICH
Intraparenchymal ICH was symptomatic in 10 (38%) patients and extraparenchymal ICH was symptomatic in 4 (8%) patients. A complete list of asymptomatic and symptomatic ICH combinations based on the Heidelberg criteria is presented in Supplemental Table 2. BP parameters did not differ significantly between patients with an asymptomatic and symptomatic intraparenchymal or extraparenchymal ICH (Table 3). In the sensitivity analysis, three additional patients with an intraparenchymal ICH were classified as symptomatic within 24 h post-EVT, which did not yield different results (Supplemental Table 8).
Missing BP Measurements
Baseline characteristics between patients with and without missing BP measurements are presented in Supplemental Table 9. None of the baseline characteristics significantly differed between the two groups. Additionally, the sensitivity analyses excluding patients with missing BP measurements, leaving a total of 366 patients, yielded highly similar results (Supplemental Table 10).
Discussion
In this retrospective single-center study, we showed an association between procedural BP and the occurrence of immediate post-EVT intraparenchymal ICH in acute stroke patients who underwent EVT. We did not find an association with extraparenchymal ICH. While extraparenchymal ICH most commonly occurs due to (mechanical) trauma of vessels, intraparenchymal ICH occurs most commonly due to ischemic damage to the cerebral blood vessels and brain tissue and this may be influenced by BP levels.
Previous studies have demonstrated that both elevated SBP pre-EVT and elevated or variable SBP in the first 24 h post-EVT are associated with the occurrence of ICH [7, 8, 25,26,27]. However, previous literature has not demonstrated a reduction in intraparenchymal ICH rate between 24 and 36 h post-EVT by active post-procedural BP lowering [28]. Considering BP during EVT, decreases in BP have been associated with worse functional outcome and larger infarct volumes [29, 30]. The literature on elevated BP and post-EVT ICH is sparser. While we found that SBPmax, SBPmean, MAPmean were associated with a higher odds of ICH, one study found that higher procedural SBPmean and MAP were associated with a lower odds of ICH post-EVT [31]. This difference in results may be partially explained by the fact the median procedural SBPmean and MAP in their cohort (128 and 91 mmHg, respectively) were lower compared to our study cohort (150 and 101 mmHg, respectively). As their admission SBP and MAP were comparable to the characteristics of our population and previously published results from larger stroke cohorts (e.g., the MR CLEAN Registry), it is possible that more BP lowering medication was used between admission and EVT in their cohort, which may have influenced their findings [31, 32]. Still, it is possible that both elevated and decreased procedural BP have a deleterious effect on ICH development post-EVT. A potential benefit of individual BP regulation may be explored in future trials.
The frequency in which ICH was observed in our study was relatively low, namely 5.4% intraparenchymal ICH, and more specifically 2.5% parenchymal hematoma type 1 and 2 compared to 14.5–17.7% parenchymal hematoma type 1 and 2 in other procedural BP and post-EVT ICH studies [4, 31, 33]. One reason for this could be different timing of the CT scans. Even though most ICH occur within hours post-EVT it is possible that immediate post-EVT underestimates the development of ICH [34]. Another reason could be the use of non-contrast CT without dual-energy technique at 24–36 h in other studies, which can overestimate the ICH occurrence. One study evaluating patients with DECT post-EVT found 59% of ICH findings were mimicked by contrast extravasation within 18 h, and 28% of ICH findings were mimicked by contrast extravasation between 18 and 36 h, suggesting that remaining hyperdensities days after the EVT procedure could still consist of contrast extravasation rather than sole hemorrhage [35].
In our study, we did not find a significant interaction term of BP with recanalization status on ICH. Previous studies indicated that elevated procedural BP before and immediately after recanalization was associated with intraparenchymal ICH post-EVT and worse functional outcome [4, 36]. While we hypothesized that in patients who achieved successful recanalization elevated BP could cause ICH due to reperfusion injury, none of the BP parameters showed an interaction with recanalization status on the occurrence of ICH. However, due to the observational design and missing data on time point of recanalization, we could not reliably distinguish between BP before and after reperfusion. Prospective trials should evaluate this interaction further, to determine if actively lowering BP after successful recanalization could decrease occurrence of ICH and improve patient outcomes.
In our study, BP parameters did not statistically differ between asymptomatic and symptomatic ICH. Moreover, our sensitivity analysis including symptomatic ICHs at 24 h which were asymptomatic at moment of DECT did not yield different results. It is hypothesized that there are inter-individual differences in BP needed to perfuse the viable penumbra; therefore, it is possible that the same elevated procedural BP could have different outcomes (e.g., asymptomatic or symptomatic ICH) in different patients. Further randomized controlled trials could shed some light in individualized procedural BP management [37, 38].
The strengths of this study include our access to a large cohort of patients who received routinely DECT post-EVT. However, some limitations need to be considered. Since DECT post-EVT is part of standard care in our center, we retrospectively had to assess whether a patient was asymptomatic or symptomatic at time of DECT. Secondly, procedural BP were collected retrospectively, which resulted in a substantial proportion of missing BP parameters. However, our sensitivity analysis excluding patients with missing BP measurements yielded highly similar results. Third, BP is highly variable, it was only recorded every three to five minutes till the end of the procedure and only three patients had invasive intra-arterial blood pressure measurements. As a result, it is possible that valuable information in-between measurements were missed as well as BP between end of the procedure and DECT. Moreover, there was no standardized BP medication protocol during EVT. Lastly, this being a single-center study, the results might not be generalizable.
Conclusion
Procedural SBPmax, SBPmean, and MAPmean are associated with intraparenchymal ICH on immediate post-EVT DECT but not with extraparenchymal ICH. Future randomized controlled trials are needed to evaluate whether individual procedural BP management can reduce post-EVT ICH and improve clinical outcome.
Abbreviations
- BP:
-
Blood pressure
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- MAP:
-
Mean arterial pressure
- EVT:
-
Endovascular treatment
- ICH:
-
Intracranial hemorrhage
- DECT:
-
Dual-energy computed tomography
- eTICI:
-
Expanded treatment in cerebral infarction
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
References
Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(1723–1731):20160218. https://doi.org/10.1016/S0140-6736(16)00163-X.
Hao Y, Zhang Z, Zhang H, et al. Risk of intracranial hemorrhage after endovascular treatment for acute ischemic stroke: systematic review and meta-analysis. Interv Neurol. 2017;6(57–64):20170119. https://doi.org/10.1159/000454721.
Balami JS, White PM, McMeekin PJ, et al. Complications of endovascular treatment for acute ischemic stroke: prevention and management. Int J Stroke. 2018;13(348–361):20171124. https://doi.org/10.1177/1747493017743051.
Cheng H, Xu C, Jin X, et al. Association of blood pressure at successful recanalization and parenchymal hemorrhage after mechanical thrombectomy with general anesthesia. Front Neurol. 2020;11(582639):20201117. https://doi.org/10.3389/fneur.2020.582639.
Samuels N, van de Graaf RA, van den Berg CAL, et al. Blood pressure during endovascular treatment under conscious sedation or local anesthesia. Neurology. 2021;96:e171–81. https://doi.org/10.1212/WNL.0000000000011006.
Maier B, Fahed R, Khoury N, et al. Association of blood pressure during thrombectomy for acute ischemic stroke with functional outcome: a systematic review. Stroke. 2019;50(2805–2812):20190829. https://doi.org/10.1161/STROKEAHA.119.024915.
Wiacek M, Szymanski M, Walewska K, et al. Blood pressure changes during mechanical thrombectomy for acute ischemic stroke are associated with serious early treatment complications: symptomatic intracerebral hemorrhage and malignant brain edema. Front Neurol. 2022;13(884519):20220705. https://doi.org/10.3389/fneur.2022.884519.
Anadani M, Orabi MY, Alawieh A, et al. Blood pressure and outcome after mechanical thrombectomy with successful revascularization. Stroke. 2019;50(2448–2454):20190718. https://doi.org/10.1161/STROKEAHA.118.024687.
Maier B, Delvoye F, Labreuche J, et al. Impact of blood pressure after successful endovascular therapy for anterior acute ischemic stroke: a systematic review. Front Neurol. 2020;11(573382):20201029. https://doi.org/10.3389/fneur.2020.573382.
Liu K, Jiang L, Ruan J, et al. The role of dual energy CT in evaluating hemorrhagic complications at different stages after thrombectomy. Front Neurol. 2020;11(583411):20201007. https://doi.org/10.3389/fneur.2020.583411.
Tijssen MP, Hofman PA, Stadler AA, et al. The role of dual energy CT in differentiating between brain haemorrhage and contrast medium after mechanical revascularisation in acute ischaemic stroke. Eur Radiol. 2014;24(834–840):20131121. https://doi.org/10.1007/s00330-013-3073-x.
Pirson F, Hinsenveld WH, Goldhoorn RB, et al. MR CLEAN-LATE, a multicenter randomized clinical trial of endovascular treatment of acute ischemic stroke in The Netherlands for late arrivals: study protocol for a randomized controlled trial. Trials. 2021;22(160):20210224. https://doi.org/10.1186/s13063-021-05092-0.
van der Steen W, van de Graaf RA, Chalos V, et al. Safety and efficacy of aspirin, unfractionated heparin, both, or neither during endovascular stroke treatment (MR CLEAN-MED): an open-label, multicentre, randomised controlled trial. Lancet. 2022;399(1059–1069):20220228. https://doi.org/10.1016/S0140-6736(22)00014-9.
DeMers D, Wachs D. Physiology mean arterial pressure. Treasure Island: StatPearls; 2022.
Del Giorno R, Balestra L, Heiniger PS, et al. Blood pressure variability with different measurement methods: reliability and predictors. A proof of concept cross sectional study in elderly hypertensive hospitalized patients. Medicine (Baltimore). 2019;98:e16347. https://doi.org/10.1097/MD.0000000000016347.
Pinckaers FM, Mentink MM, Boogaarts HD, et al. Early post-endovascular treatment contrast extravasation on dual-energy CT is associated with clinical and radiological stroke outcomes: a 10-year single-centre experience. Eur Stroke J. 2023;8:508–16. https://doi.org/10.1177/23969873231157901.
von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(2981–2986):20150901. https://doi.org/10.1161/STROKEAHA.115.010049.
Lee H, Qureshi AM, Mueller-Kronast NH, et al. Subarachnoid hemorrhage in mechanical thrombectomy for acute ischemic stroke: analysis of the STRATIS Registry, systematic review, and meta-analysis. Front Neurol. 2021;12(663058):20210525. https://doi.org/10.3389/fneur.2021.663058.
Motto C, Ciccone A, Aritzu E, et al. Hemorrhage after an acute ischemic stroke. MAST-I collaborative group. Stroke. 1999;30:761–4. https://doi.org/10.1161/01.str.30.4.761.
van Buuren S. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. https://doi.org/10.18637/jss.v045.i03.
Von Hippel PT. How many imputations do you need? A two-stage calculation using a quadratic rule. Sociol Methods Res. 2020;49:699–718. https://doi.org/10.1177/0049124117747303.
Alawieh A, Vargas J, Fargen KM, et al. Impact of procedure time on outcomes of thrombectomy for stroke. J Am Coll Cardiol. 2019;73:879–90. https://doi.org/10.1016/j.jacc.2018.11.052.
Renu A, Amaro S, Laredo C, et al. Relevance of blood-brain barrier disruption after endovascular treatment of ischemic stroke: dual-energy computed tomographic study. Stroke. 2015;46(673–679):20150205. https://doi.org/10.1161/STROKEAHA.114.008147.
Liebeskind DS, Bracard S, Guillemin F, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11(433–438):20180907. https://doi.org/10.1136/neurintsurg-2018-014127.
Kim TJ, Park HK, Kim JM, et al. Blood pressure variability and hemorrhagic transformation in patients with successful recanalization after endovascular recanalization therapy: a retrospective observational study. Ann Neurol. 2019;85(574–581):20190306. https://doi.org/10.1002/ana.25434.
Mistry EA, Mistry AM, Nakawah MO, et al. Systolic blood pressure within 24 hours after thrombectomy for acute ischemic stroke correlates with outcome. J Am Heart Assoc. 2017;6:20170518. https://doi.org/10.1161/JAHA.117.006167.
Mulder M, Ergezen S, Lingsma HF, et al. Baseline blood pressure effect on the benefit and safety of intra-arterial treatment in MR CLEAN (multicenter randomized clinical trial of endovascular treatment of acute ischemic stroke in the Netherlands). Stroke. 2017;48(1869–1876):20170421. https://doi.org/10.1161/STROKEAHA.116.016225.
Mazighi M, Richard S, Lapergue B, et al. Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP-TARGET): a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2021;20(265–274):20210226. https://doi.org/10.1016/S1474-4422(20)30483-X.
LowhagenHenden P, Rentzos A, Karlsson JE, et al. Hypotension during endovascular treatment of ischemic stroke is a risk factor for poor neurological outcome. Stroke. 2015;46(2678–2680):20150714. https://doi.org/10.1161/STROKEAHA.115.009808.
Petersen NH, Ortega-Gutierrez S, Wang A, et al. Decreases in blood pressure during thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke. 2019;50(1797–1804):20190604. https://doi.org/10.1161/STROKEAHA.118.024286.
Pikija S, Trkulja V, Ramesmayer C, et al. Higher blood pressure during endovascular thrombectomy in anterior circulation stroke is associated with better outcomes. J Stroke. 2018;20(373–384):20180930. https://doi.org/10.5853/jos.2018.01305.
Jansen IGH, Mulder M, Goldhoorn RB, et al. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN registry). BMJ. 2018;360(k949):20180309. https://doi.org/10.1136/bmj.k949.
John S, Hazaa W, Uchino K, et al. Lower intraprocedural systolic blood pressure predicts good outcome in patients undergoing endovascular therapy for acute ischemic stroke. Interv Neurol. 2016;4(151–157):20160225. https://doi.org/10.1159/000444098.
van der Steen W, van der Ende NA, van Kranendonk KR, et al. Timing of symptomatic intracranial hemorrhage after endovascular stroke treatment. Eur Stroke J. 2022;7(393–401):20220803. https://doi.org/10.1177/23969873221112279.
Almqvist H, Holmin S, Mazya MV. Dual energy CT after stroke thrombectomy alters assessment of hemorrhagic complications. Neurology. 2019;93:e1068–75. https://doi.org/10.1212/WNL.0000000000008093.
Chen M, Kronsteiner D, Pfaff J, et al. Hemodynamic status during endovascular stroke treatment: association of blood pressure with functional outcome. Neurocrit Care. 2021;35(825–834):20210617. https://doi.org/10.1007/s12028-021-01229-w.
Chen M, Kronsteiner D, Mohlenbruch MA, et al. Individualized blood pressure management during endovascular treatment of acute ischemic stroke under procedural sedation (INDIVIDUATE)—an explorative randomized controlled trial. Eur Stroke J. 2021;6(276–282):20210304. https://doi.org/10.1177/23969873211000879.
Maier B, Gory B, Chabanne R, et al. Effect of an individualized versus standard blood pressure management during mechanical thrombectomy for anterior ischemic stroke: the DETERMINE randomized controlled trial. Trials. 2022;23(598):20220726. https://doi.org/10.1186/s13063-022-06538-9.
Acknowledgements
We thank the MR CLEAN pre-trial, MR CLEAN trial, MR CLEAN Registry, MR CLEAN MED, and MR CLEAN NO-IV for providing part of the data used in this study.
Funding
None.
Author information
Authors and Affiliations
Contributions
MR was involved in study development, data collection, data analysis, and writing of the manuscript. FP was involved in gaining ethical approval, and data collection. SO was involved in study development. MB was involved in data collection. JS was involved in study development. AA was involved in study development, gaining ethical approval, and data collection. All authors contributed to the interpretation of data, reviewed and edited the manuscript, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
W.Z reports consulting fees from Philips, and speaker fees from Stryker, Cerenovus, and Nicolab (all paid to the institution). Moreover, he is participating in the Safety Monitoring Board or Advisory Board of WeTrust (Philips), InEcxtremis (CHU Montpellier), ANAIS (Anaconda), and DISTAL (University of Hospital Basal) studies. A.P received an institutional grant from Siemens Healthineers and Bayer Healthcare. Other co-authors have nothing to disclose.
Ethical Approval
Ethical approval for this study was obtained from medical ethics committee of Maastricht University Medical Centre + , Maastricht, Netherlands (METC-2020–1456).
Informed Consent
The need for individual patient consent has been waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Robbe, M.M.Q., Pinckaers, F.M.E., Olthuis, S.G.H. et al. Procedural Blood Pressure and Intracranial Hemorrhage on Dual-Energy Computed Tomography After Endovascular Stroke Treatment. Cardiovasc Intervent Radiol 47, 483–491 (2024). https://doi.org/10.1007/s00270-023-03619-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-023-03619-3