Abstract
Background
With the increasing use of magnetic resonance imaging in the assessment of acute intracerebral hemorrhage, diffusion-weighted imaging hyperintense lesions have been recognized to occur at sites remote to the hematoma in up to 40% of patients. We investigated whether blood pressure reduction was associated with diffusion-weighted imaging hyperintense lesions in acute intracerebral hemorrhage and whether such lesions are associated with worse clinical outcomes by analyzing imaging data from a randomized trial.
Methods
We performed exploratory subgroup analyses in an open-label randomized trial that investigated acute blood pressure lowering in 1000 patients with intracerebral hemorrhage between May 2011 and September 2015. Eligible participants were assigned to an intensive systolic blood pressure target of 110–139 mm Hg versus 140–179 mm Hg with the use of intravenous nicardipine. Of these, 171 patients had requisite magnetic resonance imaging sequences for inclusion in these subgroup analyses. The primary outcome was the presence of diffusion-weighted imaging hyperintense lesions. Secondary outcomes included death or disability and serious adverse event at 90 days.
Results
Diffusion-weighted imaging hyperintense lesions were present in 25% of patients (mean age 62 years). Hematoma volume > 30 cm3 was an adjusted predictor (adjusted relative risk 2.41, 95% confidence interval 1.00–5.80) of lesion presence. Lesions occurred in 25% of intensively treated patients and 24% of standard treatment patients (relative risk 1.01, 95% confidence interval 0.71–1.43, p = 0.97). Patients with diffusion-weighted imaging hyperintense lesions had similar frequencies of death or disability at 90 days, compared with patients without lesions.
Conclusions
Randomized assignment to intensive acute blood pressure lowering did not result in a greater frequency of diffusion-weighted imaging hyperintense lesion. Alternative mechanisms of diffusion-weighted imaging hyperintense lesion formation other than hemodynamic fluctuations need to be explored.
Clinical trial registration ClinicalTrials.gov (Ref. NCT01176565; https://clinicaltrials.gov/ct2/show/NCT01176565).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increasing use of magnetic resonance imaging (MRI) in the assessment of acute intracerebral hemorrhage (ICH), diffusion-weighted imaging hyperintense lesions (DWIHL) have been recognized to occur at sites remote to the hematoma in 11–41% of individuals [1,2,3,4,5,6,7,8,9,10,11,12]. DWIHLs are believed to predominantly represent acute and subacute ischemic lesions [13,14,15]. In view of their association with rapid therapeutic reductions in mean arterial pressure (MAP) [2, 6, 7], the triggering event for DWIHLs has been hypothesized to be hypoperfusion in the setting of blood pressure dysregulation in patients with ICH [11]. However, methodological limitations imposed by the observational design of all relevant studies to date hamper our current understanding of the pathogenesis of DWIHLs. For instance, residual confounding from a common indirect link between greater changes in MAP and DWIHLs [3, 4, 7] may account for the association, rather than a causal treatment effect. Consistent with this view are results reported by the Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial (ICH ADAPT) investigators, which did not show a clinically relevant reduction in cerebral blood flow or blood volume in patients with acute ICH who had rapid reduction of systolic blood pressure (SBP) to < 150 mm Hg in comparison with < 180 mm Hg [9] nor an association with blood pressure reduction and DWIHLs [10].
The Antihypertensive Treatment of Acute Cerebral Hemorrhage II (ATACH-2) clinical trial offers a unique opportunity to examine whether randomized allocation to acute intensive blood pressure lowering contributes to DWIHLs on MRI. We tested the hypothesis that the frequency of DWIHLs is not higher in the intensive blood pressure lowering group compared with standard care. We also aimed to examine whether DWIHLs predict worse outcomes in patients with ICH and whether the presence of DWIHLs modifies the effect of intensive blood pressure lowering.
Methods
Study Design
The rationale, design, and main results of the ATACH-2 randomized controlled trial have been reported elsewhere [16, 17]. The CTA spot sign score in acute cerebral hemorrhage (SCORE-IT) is a prospective observational study nested within the ATACH-2 trial, with exploratory analysis of DWIHLs in trial participants who underwent clinical brain MRI during their initial hospitalization [18].
Standard Protocol Approvals, Registrations, and Patient Consents
The ATACH-2 protocol and consent forms were approved by the institutional review board or equivalent ethics committee at each participating site, and all participants or their legally authorized representative provided written informed consent.
Study Participants
In brief, ATACH-2 was an international randomized open-label clinical trial investigating the optimal acute blood pressure target in patients with ICH. The trial was stopped for futility once 1000 of the target 1280 participants were enrolled. Eligible participants were patients aged ≥ 18 years with ICH volumes < 60 cc on computed tomography and a Glasgow Coma Scale score of 5 or more on initial assessment in whom the study drug could be initiated within 4.5 h of symptom onset. At least one reading of SBP of 180 mm Hg or more from symptom onset was required for eligibility.
ATACH-2 participants were eligible for the current analysis if they had a clinical brain MRI within 24 h and 10 days following randomization, with interpretable axial diffusion-weighted imaging (DWI) and apparent diffusion coefficient sequences.
Intervention
Eligible participants were randomly assigned (1:1) to a SBP target of 110–139 mm Hg (intensive treatment) or a target of 140–179 mm Hg (standard treatment) with the use of intravenous nicardipine. The infusion was started within 4.5 h of symptom onset.
Data Collection
Demographic information and vascular risk factors were prospectively recorded at the time of study enrollment as described in the ATACH-2 trial protocol [16, 17]. ΔMAP was calculated by subtracting the minimum MAP prior to MRI from the maximum MAP prerandomization. ΔSBP was calculated through the same equation by using the corresponding SBP values.
Imaging Acquisition and Analysis
Computed tomography and MRI images were reviewed centrally by the ATACH-2 and SCORE-IT teams. ICH topography, volume, and associated intraventricular hemorrhage were rated on computed tomography scans Obtained at entry. Intrahematomal contrast extravasation (spot sign) was rated on computed tomography angiography as previously described [18].
In addition to DWIHLs, MRI markers of interest included cerebral microbleeds, rated on gradient echo sequences or susceptibility weighted imaging according to standardized criteria [19, 20], and white matter hyperintensities (WMH), which were evaluated visually on fluid attenuated inversion recovery (FLAIR) images using the Fazekas scale [21].
DWIHLs were defined as remote areas outside of the perihematomal border that were hyperintense on DWI with either corresponding hypointensity or isointensity on apparent diffusion coefficient maps.
All MRI images were independently rated by one primary rater (AS) as well as one secondary rater (JOF, FS, AM). The primary rater (AS) has previously demonstrated excellent interrater (AS and JOF; n = 40) agreement for the presence (Κ = 0.82, p < 0.001) and number (intraclass correlation coefficient = 0.85, p < 0.001) of DWIHLs [22]. The raters were blinded to baseline features, randomization, and outcomes. Any disagreement between the primary and secondary raters were reviewed and resolved according to consensus.
Outcomes
Adjudication of all outcomes was blinded to intervention. The primary outcome was the presence of DWIHLs on MRI performed with 24 h and 10 days of randomization. Secondary outcomes of interest were (1) death or disability (defined as a modified Rankin scale of 4–6) at 90 days, (2) hematoma volume expansion of 33% or more on a computed tomography scan obtained 24 h after randomization (as compared with the entry scan), (3) neurological deterioration within 24 h (defined as a decrease from baseline of 2 or more points in the Glasgow Coma Scale score or an increase of 4 or more points in the score on the National Institutes of Health Stroke Scale) that was not related to sedation or hypnotic-agent use and was sustained for at least 8 h within the 24 h after randomization, (4) serious adverse events occurring within 90 days after randomization and that were considered by the site investigator to be related to treatment, and (5) quality of life as assessed by the European Quality of Life-5 Dimensions (EQ-5D) questionnaire. The 3-month EQ-5D utility index (on which scores range from − 0.109 [least favorable health state] to 1 [most favorable health state], with 0 imputed for death) was derived by applying Shaw’s weight to the response patterns of the five questions regarding mobility, self-care, usual activities, pain and discomfort, and anxiety and depression. The EQ-5D visual-analog scale score was obtained by requesting that patients indicate their perception of their own health state on a scale of 0 (worst) to 100 (best), with a score of 0 assigned to those who died [17].
Statistical Analysis
Patient demographic and clinical characteristics were compared between groups in cross-sectional analyses using a χ2 or Fisher’s exact test for categorical variables and Student’s t-test or Kruskal–Wallis test for continuous variables. Independent predictors of DWIHLs were assessed with a multivariable logistic regression model. Multivariable analyses adjusting for age, baseline Glasgow Coma Scale score, and presence of intraventricular hemorrhage at baseline were used to assess the association between DWIHL and (1) death and disability, (2) hematoma volume expansion, and (3) serious adverse events (SAEs). Analyses adjusting for age, baseline Glasgow Coma Scale score, presence/absence of intraventricular hemorrhage at baseline, total Fazekas Score (WMH) and time from onset to baseline computed tomography were used for the outcome of hematoma volume expansion and analyses adjusting for age, baseline Glasgow Coma Scale score, intraventricular hemorrhage at baseline, and diabetes mellitus were used for the outcome of neurological deterioration within 24 h. These covariables were selected a priori based on the known predictors of these outcomes. Treatment interaction between randomized intervention and DWIHL for the outcome of death or disability at 90 days was assessed, with analyses following the intention-to-treat paradigm. All tests were two-sided, and statistical significance was accepted at the 0.05 level. Analyses were performed with the statistical software SAS 9.4.
Data Availability Statement
Individual deidentified ATACH-2 participant data are available through the National Institute of Neurological Disorders and Stroke (NINDS) Archived Clinical Research Dataset Web site.
Results
Diffusion-weighted images were available in 171 of 1,000 (17.1%) randomized ATACH-2 participants between May 2011 and September 2015. Of those excluded, 16 (1.9%) participants were enrolled at centers where the internal review board did not approve central review of MRIs, 763 (92.0%) participants did not undergo MRI, 42 (5.1%) did not have axial DWI/apparent diffusion coefficient sequences available, 7 participants had their MRI > 10 days post randomization (0.8%), and 2 (0.2%) were excluded as the MRI suggested that the symptomatic hemorrhage was hemorrhagic transformation of an ischemic infarct, rather than primary ICH. Patients included in this cohort were less likely to be Asian American and more likely to be White, Black, and hyperlipidemic. In addition, they had less baseline neurological deficit compared with excluded ATACH-2 participants (Table 1).
Included participants had a mean age (standard deviation [SD]) of 61.8 (13.1) years and 102 (62.6%) were men. A past medical history of hypertension (n = 131, 78.0%), smoking (n = 70, 44.9%) and hyperlipidemia (n = 55, 33.5%) were common. MRI were performed within 24 h to 10 days following randomization at a median (interquartile range [IQR]) of 1.5 (0.4–4.6) days after randomization.
Twenty-five percent (n = 42) of the 171 patients had at least one DWIHL (Table 2). Patients with DWIHLs were more likely to be Black, have a prior history of stroke/transient ischemic attack, congestive heart failure, and diabetes mellitus, and tended to have greater renal insufficiency (Table 2). Minimum MAP prior to MRI was lower in patients with DWIHLs (112.2 ± 19.3 mm Hg) than those without.
(119.7 SD ± 19.7 mm Hg, p = 0.03) and there existed a numerical trend for greater maximum MAP prior to randomization (148.8 ± 19.7 vs. 142.8 ± 17.7 mm Hg, p = 0.07) in this subgroup, which resulted in greater median ΔMAP in patients with DWIHL(s) than in those without (32.7 [IQR 19–50.7] vs. 21 [IQR 7.3–34.7] mm Hg, p < 0.001). ΔSBP was also greater in patients with DWIHL(s) (40.5 [29.0, 66.0] vs. 33 [12.0, 51.0] mm Hg, p = 0.003).
Neuroimaging findings are summarized in Table 3. The median number of DWIHL lesions in each patient was 1 (IQR 1–3). DWHILs were found strictly ipsilateral to the primary hematoma in 38.1% of cases, strictly contralateral in 31%, and were bilateral in 31%. They were distributed in lobar, deep, and mixed lobar/deep regions in 47.6%, 16.7%, and 21.4% of cases, respectively. Hematoma volumes > 30 cm3 were more frequent in the groups of patients with DWIHL (17.1% vs. 4.7%, p = 0.02). There were no differences in ICH topography, presence of intraventricular hemorrhage, computed tomography angiography spot sign, or other MRI markers of small vessel disease between patients with and without DWIHLs (Table 3).
Multivariable analysis indicated only ICH volume > 30 cm3 remained independently associated with DWIHLs (Table 4). The relationship with ΔMAP in univariable analysis was not significant after adjusted analyses (Table 4). Similarly, when substituting ΔSBP for ΔMAP (due to collinearity) into this multivariable analysis the association with ΔSBP was no longer significant (adjusted relative risk [aRR] 1.01, 95% confidence interval [CI] 0.99, 1.02; p = 0.35). Further exploratory analysis assessing for potential interaction with MRI markers of cerebral small vessel disease (CSVD), demonstrated the absence of an interaction between ΔMAP and microbleed presence (p = 0.93) or with moderate to severe WMH (total Fazekas scale 3–6; p = 0.48) for the outcome of DWIHL.
Association with Randomized Intervention and Achieved Blood Pressures
The proportion of patients with DWIHLs did not differ among those allocated to intensive blood pressure treatment and those allocated to standard treatment (25% [21 of 85] vs. 24% [21 of 86]; RR 1.01, 95% CI 0.71–1.43, p = 0.97). An area under the curve analysis comparing mean hourly minimum systolic blood pressures during the first 24 h following randomization between patients with DWIHLs and those without indicated achieved minimum systolic blood pressures were similar in both groups (Fig. 1; p = 0.27).
Secondary Outcomes and Treatment Interactions
During a mean ± SD follow-up of 92.1 ± 8.5 days, 45 (28.5%) of 158 patients died or were left disabled (Modified Rankin Scale score ≥ 4); 31 (25.2%) of 123 patients without DWIHLs and 14 (40.0%) of 35 patients with DWIHLs (Table 5). Participants with DWIHLs were not at significantly increased risk of death or disability (aRR 1.59; 95% CI 0.84–3.02). Hematoma expansion was observed in 30 (20.5%) of 146 patients, 22 (19.8%) of 111 patients without DWIHLs and 8 (22.9%) of 35 patients with DWIHLs. Multivariable analysis indicated that patients with DWIHLs were not at increased risk of hematoma expansion (aRR 1.22; 95% CI 0.53–2.76). Neurological deterioration within 24 h occurred in a similar proportion of both groups; 9 of 129 (7.0%) patients without DWIHLs and 4 of 42 (9.5%) with DWIHLs (aRR 1.15; 95% CI 0.35–3.83). Any serious adverse event at 90 days occurred in 41 patients and at an approximately twofold greater rate in patients with DWIHLs, compared with those without; 25 (19.4%) of patients without DWIHLs and 16 (38.1%) of patients with DWIHLs (aRR 1.92; 95% CI 1.01–3.65). The increased risk of serious adverse events did not seem isolated to neurological events (supplementary Table 1). The median EQ-5D utility index and visual-analog scale scores were not lower at 3 months in patients with DWIHLs (Table 5).
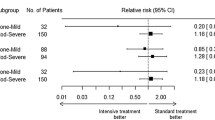
The risk of poor outcome (Modified Rankin Scale score 4–6) was similar for those assigned to intensive versus standard acute blood pressure lowering among patients with DWIHLs (aRR 1.13; 95% CI 0.39–3.29) and those without DWIHLs (aRR 1.18; 95% CI 0.56–2.48; p value for interaction = 0.98). There was no effect modification observed with number of DWIHLs (Fig. 2).
Treatment interaction between treatment assignment and DWIHL status for outcome of death and disability at 90 days. *Multivariable analysis adjusting for age, baseline Glasgow Coma Scale score and presence of intraventricular hemorrhage at baseline. CI, confidence interval, DWIHL, diffusion-weighted imaging hyperintense lesions
Discussion
Overall, we found that DWIHLs are prevalent on MRIs obtained within 24 h to 10 days following ICH in patients with ICH of mild to moderate severity who present with an acute high blood pressure. DWIHLs were associated with larger ICH volumes and greater rates of serious adverse events, but not disability, at 3 months. We found no evidence that random assignment to intensive blood pressure lowering, or actual achieved SBP, was associated with DWIHLs.
In contrast to suggestions from observational studies, our findings assessing randomized acute intensive blood pressure lowering suggest alternative contributory mechanisms over hemodynamic fluctuations as the causative trigger for DWIHLs. Although our analysis lacks the power to confidently exclude such an effect, there were no suggestive numerical trends identified. Moreover, our findings are consistent with a recent individual patent data meta-analysis that similarly noted an association between DWIHLs and baseline hematoma volume, but not with degree of blood pressure reduction [23]. It is uncertain whether the consistently reported association between DWIHLs and hematoma volume is a direct or indirect one. The observation that DWIHLs often occur rather remotely from the inciting hematoma or perihematomal region (such as in the contralateral hemisphere, or infratentorial DWIHLs in supratentorial ICH, in the absence of subfalcine or transtentorial/uncal herniation) would suggest an indirect association. The observation that DWIHLs continue to occur outside of the acute post-ICH period suggest that they are a manifestation of active underlying cerebral small vessel disease [5, 7]. One possible mechanism is that in acute ICH, the rapid release of cytokines and activation of the clotting cascade generate an inflammatory/prothrombotic milieu that promotes microthrombosis at regions of vulnerable cerebral vessels afflicted by small vessel disease. Associations between DWIHLs and larger hematoma volume, intraventricular extension, stress-induced hyperglycemia, and surgical evacuation, all of which may exacerbate these cascades, could be consistent with this hypothesis [2, 7, 24]. In our study larger hematoma volume was the sole independent predictor of DWIHLs on MRI. It should be noted that the remote punctate DWIHLs reported here are different from the rim of perihematomal DWI hyperintensity (with associated decreased apparent diffusion coefficient) that has also been reported in acute/subacute ICH. The latter has been associated with larger ICH volume, but not MRI markers of CSVD and is believed to represent cytotoxic edema [10]. It is however uncertain whether this cytotoxic edema results from ischemia or alternate mechanisms, such as hematoma-induced mechanical and inflammatory injury to surrounding neurons, as positron emission tomography studies have suggested that the reduced blood flow in the perihematomal region is driven by hypometabolism within this brain tissue rather than being ischemic in nature [25].
In contrast to results from the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study [11], we did not find an adjusted relationship between DWIHLs and worse outcomes in patients with ICH, although serious adverse events were more common. In view of the lack of association with neurological deterioration at 24 h, the presence of DWIHLs likely marks a more vulnerable disease population at risk for serious adverse events, rather than any direct causal relationship. The association could have also been merely a chance finding due to multiple testing.
Our results were limited by the fact that only 171 of 1,000 ATACH-2 trial participants had the requisite MRI sequences for our analyses and by the trial’s eligibility criteria, which limit the generalizability of our findings to all ICH. MRI studies in patients with stroke are prone to selection bias, and this is likely also true in an acute ICH population. Indeed, included participants were less likely to be Asian, were more hyperlipidemic and had less neurological deficit at baseline compared with excluded participants. Similar selection bias—particularly in relation to neurological status—likely applies to previous ICH MRI studies on DWIHLs. Trial eligibility mandated an acute blood pressure > 179 mm Hg, which likely selected against patients with cerebral amyloid angiopathy who typically present with lower blood pressures [26]. Fittingly, our mean age 62 years (although similar to excluded participants in ATACH-2) is younger than most ICH cohorts. In ATACH-2, mean achieved systolic blood pressures within the first 2 h were 141 mm Hg in the control arm and 129 mm Hg in the intensive treatment arm, which limits our ability to compare the contribution of intensive blood pressure lowering (< 140 mm Hg) to DWIHLs relative to higher mean SBPs within the guideline treatment (140–180 mm Hg) range. The lack of variability of MRI markers of small vessel disease (i.e., > 70% with cerebral microbleeds) and rating of WMH using the visual Fazekas scale, rather than volumetric measurements, may have limited our ability to detect an association between DWIHLs and other MRI markers. Heterogeneity in sequence acquisition parameters and MRI field strengths across participating centers could have led to heterogeneous detection DWIHL detection rates and influenced our findings. Lastly, our study may not have been adequately powered to detect an association between DWIHLs and functional outcomes.
Conclusions
Randomized allocation to intensive acute blood pressure lowering did not result in greater DWIHL frequency. Accordingly, alternative mechanisms of DWIHL formation other than hemodynamic fluctuations need to be explored.
References
Kimberly WT, Gilson A, Rost NS, Rosand J, Viswanathan A, Smith EE, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72:1230–5.
Prabhakaran S, Gupta R, Ouyang B, John S, Temes RE, Mohammad Y, et al. Acute brain infarcts after spontaneous intracerebral hemorrhage: a diffusion-weighted imaging study. Stroke. 2010;41:89–94.
Gregoire SM, Charidimou A, Gadapa N, Dolan E, Antoun N, Peeters A, et al. Acute ischaemic brain lesions in intracerebral haemorrhage: multicentre cross-sectional magnetic resonance imaging study. Brain. 2011;134:2376–86.
Kang DW, Han MK, Kim HJ, Yun SC, Jeon SB, Bae HJ, et al. New ischemic lesions coexisting with acute intracerebral hemorrhage. Neurology. 2012;79:848–55.
Auriel E, Gurol ME, Ayres A, Dumas AP, Schwab KM, Vashkevich A, et al. Characteristic distributions of intracerebral hemorrhage-associated diffusion-weighted lesions. Neurology. 2012;79:2335–41.
Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood pressure reduction, decreased diffusion on mri, and outcomes after intracerebral hemorrhage. Stroke. 2012;43:67–71.
Menon RS, Burgess RE, Wing JJ, Gibbons MC, Shara NM, Fernandez S, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol. 2012;71:199–205.
Arsava EM, Kayim-Yildiz O, Oguz KK, Akpinar E, Topcuoglu MA. Elevated admission blood pressure and acute ischemic lesions in spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2013;22:250–4.
Butcher KS, Jeerakathil T, Hill M, Demchuk AM, Dowlatshahi D, Coutts SB, et al. The intracerebral hemorrhage acutely decreasing arterial pressure trial. Stroke. 2013;44:620–6.
Gioia LC, Kate M, Choi V, Sivakumar L, Jeerakathil T, Kosior J, et al. Ischemia in intracerebral hemorrhage is associated with leukoaraiosis and hematoma volume, not blood pressure reduction. Stroke. 2015;46:1541–7.
Kidwell CS, Rosand J, Norato G, Dixon S, Worrall BB, James ML, et al. Ischemic lesions, blood pressure dysregulation, and poor outcomes in intracerebral hemorrhage. Neurology. 2017;88(8):782–8.
Tsai YH, Lee MH, Weng HH, Chang SW, Yang JT, Huang YC. Fate of diffusion restricted lesions in acute intracerebral hemorrhage. PLoS One. 2014;9:e105970.
van Veluw SJ, Shih AY, Smith EE, Chen C, Schneider JA, Wardlaw JM, et al. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol. 2017;16:730–40.
van Veluw SJ, Lauer A, Charidimou A, Bounemia N, Xiong L, Boulouis G, et al. Evolution of dwi lesions in cerebral amyloid angiopathy: evidence for ischemia. Neurology. 2017;89:2136–42.
Wu B, Yao X, Lei C, Liu M, Selim MH. Enlarged perivascular spaces and small diffusion-weighted lesions in intracerebral hemorrhage. Neurology. 2015;85:2045–52.
Qureshi AI, Palesch YY. Antihypertensive treatment of acute cerebral hemorrhage (atach) ii: design, methods, and rationale. Neurocrit Care. 2011;15:559–76.
Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375:1033–43.
Goldstein J, Brouwers H, Romero J, McNamara K, Schwab K, Greenberg S, et al. Score-it: the spot sign score in restricting ich growth horizontal line an atach-ii ancillary study. J Vasc Interv Neurol. 2012;5:20–5.
Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–74.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38.
Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, et al. A new rating scale for age-related white matter changes applicable to mri and ct. Stroke. 2001;32:1318–22.
Oliveira-Filho J, Ay H, Shoamanesh A, Park KY, Avery R, Sorgun M, et al. Incidence and etiology of microinfarcts in patients with ischemic stroke. J Neuroimaging. 2018;28:406–11.
Murthy SB, Cho SM, Gupta A, Shoamanesh A, Navi BB, Avadhani R, et al. A pooled analysis of diffusion-weighted imaging lesions in patients with acute intracerebral hemorrhage. JAMA Neurol. 2020;77:1–9.
Ye XH, Cai XL, Nie DL, Chen YJ, Li JW, Xu XH, et al. Stress-induced hyperglycemia and remote diffusion-weighted imaging lesions in primary intracererbal hemorrhage. Neurocrit Care. 2020;32:427–36.
Zazulia AR, Diringer MN, Videen TO, Adams RE, Yundt K, Aiygari V, et al. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2001;21:804–10.
Shoamanesh A, Morotti A, Romero JM, Oliveira-Filho J, Schlunk F, Jessel MJ, et al. Cerebral microbleeds and the effect of intensive blood pressure reduction on hematoma expansion and functional outcomes: a secondary analysis of the atach-2 randomized clinical trial. JAMA Neurol. 2018;75:850–9.
Author information
Authors and Affiliations
Consortia
Contributions
All authorship requirements have been met, and the final manuscript was approved by all authors. Study conception and design: AS, CC, AIQ, JR, JNG. Acquisition of data: AS, CC, AM, JR, JOF, FS, MJ, AA, AV, MRA, RHM. Administrative support: MJ, AA, AV, KS. Statistical analysis and interpretation of data: AS, CC, RHM, JR, JNG. Drafting of manuscript: AS. Critical revisions: AS, CC, AM, JR, JOF, FS, MJ, KB, LG, AA, AV, MRA, KS, MRA, RHM, AIQ, SMG, JR, JNG. Study supervision/funding: JNG, JR, AIQ.
Corresponding author
Ethics declarations
Source of support
Supported by grants (National Institutes of Health - National Institute of Neurological Disorders and Stroke (NINDS) R01NS073344, to Dr. Rosand and U01-NS062091, to Dr. Qureshi). Chiesi USA and Astellas Pharma supplied intravenous nicardipine for use during the trial but had no other role in the design or conduct of the ATACH-2 trial or in this analysis/article. The National Institutes of Health, Chiesi USA, and Astellas Pharma had no involvement in the design, analyses, interpretation, or drafting of this report.
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval/informed consent
The ATACH-2 protocol and consent forms were approved by the institutional review board or equivalent ethics committee at each participating site, and all participants or their legally authorized representative provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shoamanesh, A., Cassarly, C., Morotti, A. et al. Intensive Blood Pressure Lowering and DWI Lesions in Intracerebral Hemorrhage: Exploratory Analysis of the ATACH-2 Randomized Trial. Neurocrit Care 36, 71–81 (2022). https://doi.org/10.1007/s12028-021-01254-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01254-9