Abstract
Objective
To compare the safety and effectiveness between bronchial artery embolisation (BAE) and conservative treatment for bronchiectasis-related nonmassive haemoptysis patients.
Materials and Methods
From January 2015 to December 2020, consecutive bronchiectasis-related nonmassive haemoptysis patients who underwent either BAE (n = 98) or conservative treatment (n = 118) were included. Treatment-related complications, length of hospital stays, clinical success rate, patient satisfaction, and recurrence-free survival rates were compared between groups. Prognostic factors related to recurrence were also analysed.
Results
During a median follow-up time of 44.8 months (range, 2.4–83.6 months), 34 and 66 patients in the BAE and conservative treatment groups suffered relapse. The 1-year, 2-year, 3-year and 5-year haemoptysis-free survival rates in the BAE and conservative treatment groups were 79.2%, 68.1%, 62.8%, and 57.6% and 64.0%, 52.8%, 44.1%, and 37.0%, respectively (P = 0.007). The minor complication rate after BAE was higher than that after conservative treatment (23/98 vs. 12/118, P = 0.008). BAE was associated with shorter hospital stays (5.0 vs. 7.0 days, P = 0.042) and higher patient satisfaction (88.8% vs. 74.6%, P = 0.008) than those for conservative treatment and with comparable clinical success rates (95.9% vs. 91.5%, P = 0.192). Treatment type, haemoptysis duration, and bronchiectasis severity were independently significant predictors of recurrence for these patients.
Conclusions
BAE could be another option for bronchiectasis-related nonmassive haemoptysis patients. In the patients with longer duration and more severe bronchiectasis, BAE still appeared to have better long-term haemoptysis control than conservative therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bronchiectasis refers to a chronic respiratory disease characterised by permanent bronchial dilatation [1]. Over half of bronchiectasis patients present without a definite cause and are then defined as having idiopathic disease [2, 3]. The long-term outcomes of these patients depend on various accompanying symptoms, e.g. haemoptysis, chest discomfort and tiredness [4]. Haemoptysis tends to recur with an incidence of approximately 40% [5, 6], which requires adequate assessment and effective treatment.
The widely used definition of massive haemoptysis is bleeding over 300 mL/24 h resulting in cardiorespiratory failure [7, 8]. Conversely, nonmassive haemoptysis includes mild and moderate haemoptysis. Bronchial artery embolisation (BAE) is recommended as an effective haemostasis procedure [9, 10] and is widely used for massive haemoptysis [11,12,13,14]. In contrast, nonmassive haemoptysis is considered an indication for conservative treatment.
Nevertheless, periodically recurrent haemoptysis and declining psychological status after each conservative treatment have become inevitable complaints among these patients [15]. Inadvertently, some studies have indicated that early BAE had the potential to reduce recurrence and improve quality of life (QoL) for these patients [15,16,17]. However, the prognosis and long-term outcome of BAE for these nonmassive haemoptysis patients still need to be confirmed.
Hence, we conducted this single-centre retrospective study to compare the effectiveness and safety between BAE and conservative treatment for these bronchiectasis-related nonmassive haemoptysis patients. The prognostic factors related to recurrence were also analysed.
Materials and Methods
This single-centre retrospective study was approved by the local institutional ethics review board. Ethics Committee discussed and determined that the requirement for informed consent from the patients was waived due to the retrospective nature. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The data analysed in this study are available upon reasonable request for cooperation, secondary analysis, or further exploration.
Study Participants
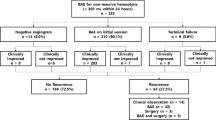
From January 2015 to December 2020, 422 consecutive adult patients aged 18 years or over with haemoptysis due to bronchiectasis presented in our institution. Among them, the medical records of 412 patients undergoing either BAE (n = 218) or conservative treatment (n = 194) were queried in this study. Ultimately, 98 patients undergoing BAE and 118 patients with conservative treatment were enrolled in this study. Detailed in/exclusion criteria are displayed in Fig. 1. In this study, there were overlapping population with our previous researches (64 patients diagnosed as nonmassive haemoptysis and 42 patients suffering frequent haemoptysis, respectively) [18, 19]. After admission, chest radiography, complete blood cell counts, and coagulation tests were completed for all patients. Bronchiectasis was diagnosed on the basis of (a) clinical symptoms, productive cough, mucopurulent sputum and protracted respiratory infections [1, 4], and (b) radiological findings, permanent dilatation of the bronchia, with cross-sectional inner diameter larger than that of the concomitant artery (inner airway-artery ratio > 1.0) on computed tomography (CT) imaging [20]. The severity of idiopathic bronchiectasis was assessed by the bronchiectasis radiologically indexed CT score (BRICS) [21], which was developed from bronchial dilatation and the number of segments with emphysema. The score ranges from 0 to 5 (0 points: absent, 1 point: mild, 2–3 points: moderate, and 4–5 points: severe). Thickness > 3 mm was the diagnostic criterion for pleural thickening [22]. Lung destruction is considered irreversible parenchymal damage characterised by extensive adhesions, cavities and lung volume loss [23].
Conservative Treatment
There was a multidisciplinary team for haemoptysis management in our institution, including the Emergency, Radiology, Respiratory Medicine, Interventional Radiology, Thoracic Surgery, and Rehabilitation Departments. Vital sign monitoring, airway stabilisation, correction of hypoxemia, haemostasis medicine, nebulised treatment, and antibiotic therapy were standard conservative therapies for each patient. Antihaemorrhagic drugs included carbazochrome sodium sulphonate, pituitrin and phentolamine. Patients were initially prescribed empirical antibiotics that were then adjusted according to culture results. Subsequent oral antibiotics were prescribed for another week after discharge.
Arterial Embolisation Procedures
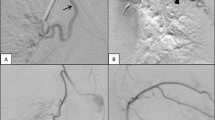
The decision of embolisation was based on invalid conservative treatment and possibility of benefit from BAE according to the positive signs on CT angiography (CTA) findings, plus informed consent of patients. The parameters of CTA included injecting dose of 50–60 mL, rate of 4.0 mL/s, and slice thickness of 1.25 mm. The culprit vessels on CTA were identified upon hypertrophy, hypervascularity, tortuosity, and aneurysmal formation of the culprit artery. We searched bronchial arteries from the proximal descending thoracic aorta and non-bronchial systemic arteries (NBSAs) were also evaluated according to the location of lung lesion. According to CTA findings, a 5F angiographic catheter (Cobra, RLG, MIK; Cook, USA) was used to select the bronchial artery and NBSAs via the femoral artery approach. The culprit vessels were identified upon hypertrophy, hypervascularity, systemic arterial-pulmonary circulation shunts (SPSs), or contrast extravasation [9]. After angiography, a microcatheter (2.7F; Terumo, Tokyo, Japan; or 2.4F; Merit Maestro, Utah, USA) was introduced coaxially and advanced as distally as possible to prevent ectopic embolisation. The agent for embolisation included polyvinyl alcohol (PVA) particles (300–500 μm; Cook, USA), microspheres (300–500 μm, 500–700 μm, Merit Maestro) and gelatin sponge particles (350–560 μm; Hangzhou Alicon Pharmaceutical Co., Ltd., Zhejiang, China). Some cases were embolised with additional microcoil (Cook, USA). Technical success of BAE was defined as complete occlusion of all culprit vessels during a single procedure [24]. Angiographic data, including the number and diameter of culprit bronchial arteries, the presence of NBSAs and SPSs, and embolisation materials, were recorded.
Follow-Up and Treatment Assessment
Outpatient clinic follow-up was scheduled at the first month after discharge. Subsequently, patients were followed up by telephone interview once every 6 months for the first 2 years and then once per year for patients without recurrence. The end date of follow-up was July 2021 or the date of death. Follow-up contents were the general conditions of patients, patient satisfaction with treatment, details of recurrence (date, bleeding volume, and treatment measures) and healthy lifestyle education. During the first follow-up, patient satisfaction was evaluated by a standardised questionnaire (Supplemental Table 1), which was referred to The St. George’s Respiratory Questionnaire [25], but simplified and advised. Recurrence was defined as relapse during follow-up requiring unscheduled admission for haemoptysis, including conservative treatment, BAE, surgical treatment, or death. Compared with the volume of haemoptysis before treatment, recurrence was semiquantitatively divided into three categories: deterioration, similarity, and improvement. Patients who suffered haemoptysis recurrence or bronchiectasis aggravation were referred to the outpatient clinic for further evaluation and treatment. We also prescribed low-dose macrolides to patients to control pulmonary infections.
Treatment assessment included treatment complications, length of hospital stays, clinical success, recurrence-free survival time, and patient satisfaction. Complications were graded according to the CIRSE classification [24]. Clinical success reflected the resolution of haemoptysis within 30 days after treatment [26]. Recurrence-free time was the period from the date of discharge to the date of recurrence, death, or last follow-up. Variables in the analysis of prognostic factors included age, sex, haemoptysis duration, smoking, hypertension, white blood cell (WBC) count, neutrophil ratio (NE%), platelet counts (PLT), prothrombin time (PT), activated partial thromboplastin time (APTT), bronchiectasis severity, pleural thickening presence, lung destruction, and treatment type.
Statistical Analysis
Continuous variables were reported as either the mean ± standard deviation (SD) or median with interquartile range (IQR), as appropriate. Categorical variables are presented as counts with percentages. Baseline characteristics of patients in the two groups were compared, the χ2 test or Fisher’s exact test for categorical variables and the t test or Wilcoxon test for continuous variables. Time-to-recurrence curves were plotted by the Kaplan–Meier method and compared using the log-rank test. Prognostic factors for recurrence were analysed with univariate and multivariate Cox proportional hazards models. Statistical analysis was performed by using SPSS (Statistical Package for the Social Sciences, version 22.0, Armonk, NY, USA). A two-tailed P value < 0.05 was considered statistically significant.
Results
Characteristics of the Study Participants
The baseline characteristics of the study population are summarised in Table 1. Patients in the BAE group had a longer haemoptysis duration (P < 0.001), higher inflammation indicator levels (WBC: P < 0.001; NE%: P = 0.001), and more severe bronchiectasis (P = 0.001) than those in the conservative treatment group.
Conservative Treatment and BAE
In total, all the patients received antibiotics with nebuliser treatments until discharge. The application of antibiotics after admission was as follows: third-generation cephalosporins and/or quinolones (n = 160), third-generation cephalosporins and azithromycin (n = 21), penicillins and/or quinolones (n = 45). Then, antibiotics were upgraded to carbapenems in 22 patients and adjusted to antifungal drugs in 3 patients. In addition, twenty-seven (12.5%) patients underwent bronchoscopy during hospitalisation.
Two hundred thirty-seven culprit arteries for a median of 2 arteries per-patient (range, 1–5), including 217 bronchial arteries and 20 NBSAs, were identified and embolised during the procedures. The technical success rate was 100%. The mean diameters of culprit bronchial arteries and NBSAs were 2.9 ± 1.1 mm and 3.3 ± 1.0 mm, respectively. SPSs were observed in 40 (40.8%) patients. Embolisation materials were PVA particles in 94 patients, microspheres in 2 patients and gelatin sponge particles in 2 patients. Microcoils were additionally used for the high-level shunts (n = 4) and aneurysmal dilatation of the culprit arteries (n = 2).
Treatment Assessments
In the BAE group, complications of Grade 2 included chest or shoulder pain (n = 13), fever (n = 6), abdominal pain (n = 3) and complications of Grade 3 included puncture site discomfort (n = 1). While stomach discomfort (n = 4), pruritus (n = 3), mild nausea (n = 3) and dizziness (n = 2) were observed in the conservative treatment group. The complication rate was higher in the BAE group than in the conservative treatment group (23/98 vs. 12/118, P = 0.008). In addition, compared with those with conservative therapy, a higher patient satisfaction rate (88.8% vs. 74.6%, P = 0.008), shorter hospital stays (5.0 days; IQR: 4.0–7.0 days vs. 7.0 days; IQR: 5.0–9.0 days, P = 0.042), and comparable clinical success rate (95.9% vs. 91.5%, P = 0.192) were also obtained with BAE. Here, patient satisfaction was evaluated by five questions in the questionnaire, all “yes” represented that this patient was satisfied with the treatment.
During a median follow-up period of 44.8 months (range, 2.4–83.6 months), 34 (34.7%) patients in the BAE group and 66 (55.9%) patients in the conservative treatment group experienced recurrent haemoptysis. The 1-year, 2-year, 3-year and 5-year haemoptysis-free survival rates of the BAE and conservative treatment groups were 79.2%, 68.1%, 62.8%, and 57.6% and 64.0%, 52.8%, 44.1%, and 37.0%, respectively (P = 0.007) (Fig. 2). Comparing the volume of haemoptysis before treatment, the relapse severity was divided into deterioration (n = 5, 14.7%), similarity (n = 9, 26.5%), and improvement (n = 20, 58.8%) in the BAE group, with n = 13 (19.7%), n = 43 (65.2%), and n = 10 (15.1%) in the conservative treatment group. Treatments or outcomes for recurrence in the conservative treatment group included medical therapy (n = 58, 87.9%), BAE (n = 5, 7.6%), segmentectomy (n = 2, 3.0%), and death due to haemoptysis (n = 1, 1.5%). In the BAE group, 19 (55.9%) patients received medical therapy, 13 (38.2%) patients underwent repeated BAE, 1 (2.9%) patient underwent segmentectomy, and 1 (2.9%) patient died of haemoptysis. The reasons for recurrence who receiving repeated BAE were missed culprit arteries (n = 1, 7.7%), new collateral artery formation (n = 5, 38.5%) and recanalisation (n = 7, 53.8%).
Univariate and multivariate analyses of predictors related to recurrence are shown in Table 2. Univariate analysis showed that the haemoptysis duration, bronchiectasis severity, lung destruction, and treatment type were statistically significant factors associated with recurrence. Then, multivariate analysis revealed that the duration of haemoptysis, bronchiectasis severity, and treatment type were independently significant prognostic factors of recurrence for these patients (all P < 0.001).
Discussion
Given the possibility of periodically recurrent haemoptysis and declining psychological status [15], we conducted this retrospective comparative study, and the results showed that the BAE could offer a superior long-term outcome and provide an alternative treatment modality for bronchiectasis-related nonmassive haemoptysis patients.
For nonmassive haemoptysis patients, the concerns regarding the BAE procedure were feasibility and safety. First, with the guidance of preoperative CTA, we could precisely identify and locate each culprit vessel. The technical success of embolisation was 100%, which also confirmed the feasibility, as reported [7, 9, 16, 17]. Second, in terms of safety, although the complication rate of the BAE procedure was higher than that of conservative treatment, complications were manageable and did not delay discharge. In contrast, the rapid and effective haemostasis accompanied by the BAE procedure reduced the length of hospital stays compared with those of the conservative treatment group in our study. Thus, BAE was also feasible and safe for such a specific population.
Recurrence after successful BAE remains an inevitable problem and occurs in approximately 30% of patients [17, 27,28,29]. In this study, the comparison of baseline characteristics revealed that patients in the BAE group had the disadvantage of a longer haemoptysis duration and suffered more severe infections and bronchiectasis. However, the results still showed that BAE maintained a better long-term outcome than conservative therapy alone, embodied in fewer recurrent events, decreased recurrent haemoptysis severity and higher patient satisfaction. In practice, bronchial arteries and NBSAs are frequent sources of haemoptysis [7,8,9]. Although BAE is considered a palliative and symptomatic treatment, it can occlude the source of bleeding, destroy abnormal vascular remodelling and postpone haemoptysis progression. In contrast, the development of new fragile bronchial vasculature and the remodelling of existing vessels are promoted continuously by chronic inflammation in bronchiectasis patients treated conservatively [30]. And SPSs around bronchus open pathologically to compensate for decreased lung perfusion, which also induce arterial hypertrophy [31]. Under systemic arterial pressure, these hypertrophic and tortuous bronchial arteries or NBSAs and SPSs may become prominent and prone to rupture. Therefore, BAE is superior to conservative treatment in reducing the recurrence probability of nonmassive haemoptysis. A Cox proportional hazards model was applied to adjust the differences between the two groups and further confirmed that BAE was a protective factor for long-term haemoptysis-free survival time for these patients (P < 0.001).
Aside from the choice of treatment type, the duration of haemoptysis and bronchiectasis severity was also related to recurrence in this study. The pathophysiological process of bronchiectasis is persistent and progressive, including infections, dysregulated inflammatory responses, impaired mucociliary clearance, and airway dysfunction [1, 32]. In the majority of patients, the progression of bronchiectasis was accompanied by the exacerbation of chronic inflammation and induced recurrent haemoptysis [15]. Therefore, after BAE procedure, patients should receive subsequent conservative treatment, including regular follow-up, medication guidance, and healthy lifestyle education, to maximally reduce pulmonary exacerbations.
There were several limitations of this study. First, this study was a retrospective design based on data from a single centre, which inevitably caused bias. Second, our study focused on these patients with haemoptysis due to idiopathic bronchiectasis. Therefore, the reliability and universality of the conclusion may be restricted regarding bronchiectasis caused by other aetiologies. Third, given the compliance of patients during follow-up, the assessment of QoL was simplified.
In conclusion, BAE could be another option for bronchiectasis-related nonmassive haemoptysis patients. In the patients with longer duration and more severe bronchiectasis, BAE still appeared to have better long-term haemoptysis control, shorter hospital stays, and higher patient satisfaction than conservative therapy.
References
Chalmers JD, Chang AB, Chotirmall SH, et al. Bronchiectasis. Nat Rev Dis Primers. 2018;4:45. https://doi.org/10.1038/s41572-018-0042-3.
Guan WJ, Gao YH, Xu G, et al. Aetiology of bronchiectasis in Guangzhou. South China Respirol. 2015;20:739–48. https://doi.org/10.1111/resp.12528.
Pasteur MC, Bilton D, Hill AT, et al. British thoracic society guideline for non-CF bronchiectasis. Thorax. 2010;65:i1–58. https://doi.org/10.1136/thx.2010.136119.
Quint JK, Smith MP. Paediatric and adult bronchiectasis: diagnosis disease burden and prognosis. Respirology. 2019;24:413–22. https://doi.org/10.1111/resp.13495.
King PT, Holdsworth SR, Freezer NJ, et al. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med. 2006;100:2183–9. https://doi.org/10.1016/j.rmed.2006.03.012.
Nicotra MB, Rivera M, Dale AM, et al. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest. 1995;108:955–61. https://doi.org/10.1378/chest.108.4.955.
Panda A, Bhalla AS, Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol. 2017;23:307–17. https://doi.org/10.5152/dir.2017.16454.
Chun JY, Morgan R, Belli AM. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol. 2010;33:240–50. https://doi.org/10.1007/s00270-009-9788-z.
Davidson K, Shojaee S. Managing massive hemoptysis. Chest. 2020;157:77–88. https://doi.org/10.1016/j.chest.2019.07.012.
Kathuria H, Hollingsworth HM, Vilvendhan R, et al. Management of life-threatening hemoptysis. J Intensive Care. 2020;8:23. https://doi.org/10.1186/s40560-020-00441-8.
van den Heuvel MM, Els Z, Koegelenberg CF, et al. Risk factors for recurrence of haemoptysis following bronchial artery embolisation for life-threatening haemoptysis. Int J Tuberc Lung Dis. 2007;11:909–14 (PMID: 17705959).
Anuradha C, Shyamkumar NK, Vinu M, et al. outcomes of bronchial artery embolization for life-threatening hemoptysis due to tuberculosis and post-tuberculosis sequelae. Diagn Interv Radiol. 2012;18:96–101. https://doi.org/10.4261/1305-3825.DIR.3876-11.2.
Pei R, Zhou Y, Wang G, et al. Outcomes of bronchial artery embolization for life-threatening hemoptysis secondary to tuberculosis. Plos One. 2014;9:e115956. https://doi.org/10.1371/journal.pone.0115956.
Baltacioğlu F, Cimşit NC, Bostanci K, et al. Transarterial microcatheter glue embolization of the bronchial artery for life-threatening hemoptysis: technical and clinical results. Eur J Radiol. 2010;73:380–4. https://doi.org/10.1016/j.ejrad.2008.10.017.
Antonelli M, Midulla F, Tancredi G, et al. Bronchial artery embolization for the management of nonmassive hemoptysis in cystic fibrosis. Chest. 2002;121:796–801. https://doi.org/10.1378/chest.121.3.796.
Choi J, Baik JH, Kim CH, et al. Long-term outcomes and prognostic factors in patients with mild hemoptysis. Am J Emerg Med. 2018;36:1160–5. https://doi.org/10.1016/j.ajem.2017.11.053.
Hwang JH, Kim JH, Park S, et al. Feasibility and outcomes of bronchial artery embolization in patients with non-massive hemoptysis. Respir Res. 2021;22:221. https://doi.org/10.1186/s12931-021-01820-x.
Yan HT, Lu GD, Huang XZ, et al. A nomogram to predict recurrence after bronchial artery embolization for hemoptysis due to bronchiectasis. Cardiovasc Intervent Radiol. 2021;44:1609–17. https://doi.org/10.1007/s00270-021-02923-0.
Lu GD, Yan HT, Zhang JX, et al. Bronchial artery embolization for the management of frequent hemoptysis caused by bronchiectasis. BMC Pulm Med. 2022;22:394. https://doi.org/10.1186/s12890-022-02198-2.
Tiddens HAWM, Meerburg JJ, van der Eerden MM, et al. The radiological diagnosis of bronchiectasis: what’s in a name? Eur Respir Rev. 2020;29:190120. https://doi.org/10.1183/16000617.0120-2019.
Bedi P, Chalmers JD, Goeminne PC, et al. The BRICS (bronchiectasis radiologically indexed CT score): a multicenter study score for use in idiopathic and postinfective bronchiectasis. Chest. 2018;153:1177–86. https://doi.org/10.1016/j.chest.2017.11.033.
Yoon W, Kim YH, Kim JK, et al. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology. 2003;227:232–8. https://doi.org/10.1148/radiol.2271020324.
Sayir F, Ocakcioglu I, Şehitoğulları A, et al. Clinical analysis of pneumonectomy for destroyed lung: a retrospective study of 32 patients. Gen Thorac Cardiovasc Surg. 2019;67:530–6. https://doi.org/10.1007/s11748-018-01055-6.
Kettenbach J, Ittrich H, Gaubert JY, et al. CIRSE standards of practice on bronchial artery embolisation. Cardiovasc Intervent Radiol. 2022;45:721–32. https://doi.org/10.1007/s00270-022-03127-w.
Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George’s respiratory questionnaire. Am Rev Respir Dis. 1992;145:1321–7. https://doi.org/10.1164/ajrccm/145.6.1321.
Dariushnia SR, Redstone EA, Heran MKS, et al. Society of interventional radiology quality improvement standards for percutaneous transcatheter embolization. J Vasc Interv Radiol. 2021;32:476.e1-476.e33. https://doi.org/10.1016/j.jvir.2020.10.022.
Kato A, Kudo S, Matsumoto K, et al. Bronchial artery embolization for hemoptysis due to benign diseases: immediate and long-term results. Cardiovasc Intervent Radiol. 2000;23:351–7. https://doi.org/10.1007/s002700010062.
Lee JH, Kwon SY, Yoon HI, et al. Haemoptysis due to chronic tuberculosis Vs. bronchiectasis: comparison of long-term outcome of arterial embolisation. Int J Tuberc Lung Dis. 2007;11:781–7.
Lu GD, Zhang JX, Zhou CG, et al. Arterial embolization for hemoptysis in patients with chronic pulmonary tuberculosis and in patients with bronchiectasis. Acta Radiol. 2019;60:866–72. https://doi.org/10.1177/0284185118805258.
McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–45. https://doi.org/10.1164/ajrccm.164.supplement_2.2106065.
McCullagh A, Rosenthal M, Wanner A, et al. The bronchial circulation-worth a closer look: a review of the relationship between the bronchial vasculature and airway inflammation. Pediatr Pulmonol. 2010;45:1–13. https://doi.org/10.1002/ppul.21135. (PMID: 20025051).
Polverino E, Goeminne PC, McDonnell MJ, et al. European respiratory society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50:1700629. https://doi.org/10.1183/13993003.00629-2017.
Funding
This study was funded by Jiangsu Province’s Key Talents Program (QNRC2016559 to Qing-Quan Zu) and Construction Program of Jiangsu Province Clinical Research Center Support System (BL2014084 to Qing-Quan Zu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional (Ethical review no. 2018-SR-097) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this retrospective study, formal consent was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, HT., Lu, GD., Zhang, JX. et al. Comparison of Bronchial Artery Embolisation Versus Conservative Treatment for Bronchiectasis-Related Nonmassive Haemoptysis: A Single-Centre Retrospective Study. Cardiovasc Intervent Radiol 46, 369–376 (2023). https://doi.org/10.1007/s00270-023-03361-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-023-03361-w