Abstract
Purpose
To assess the efficacy of percutaneous techniques in managing paediatric liver transplantation complications.
Material and Methods
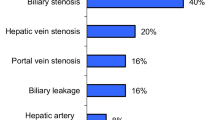
We carried out 105 paediatric cadaveric donor liver transplantations at our centre from 2001 to 2018. Percutaneous techniques were used to treat 25 cases involving transplantation complications in 23 patients. Biliary complications were treated in 14 cases (13.3%): 10 patients had bile duct obstruction, and 4 had biliary leaks. Vascular complications were treated in 11 cases (10.5%): 5 hepatic artery (HA) stenoses/occlusions, 2 inferior vena cava (IVC) stenoses, and 1 portal vein (PV) stenosis. Other interventions involved embolisation of the superior mesenteric artery branch to manage gastrointestinal bleeding in 2 patients and embolisation of an arteriobiliary fistula in 1 patient.
Results
Biliary: We carried out external–internal drainage and balloon dilatation of stenoses in 12 cases. The external–internal drainage catheter was removed after 6–8 weeks in 7 patients, with the remaining 5 patients with persisting stenosis assigned for retransplantation. We failed to cross anastomotic occlusions in 2 patients before completing the procedures using external drainage; both individuals subsequently underwent retransplantation. Vascular: We performed PTA/stenting of HA stenoses/occlusions in 4 out of 5 patients. After the procedure, all 4 patients showed liver function normalisation. All 3 cases of embolisation were technically and clinically successful. Both IVC and PV stenoses treated with dilatation/stenting were also successful.
Conclusions
Percutaneous techniques used to treat biliary and vascular complications after liver transplantation in paediatric patients are safe and efficient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver transplantation (LT) in paediatric patients is an effective therapeutic option in end-stage liver disease, delivering impressive long-term graft and patient survival outcomes [1]. However, the rate of complications following liver transplantation is higher in children compared to adult patients [2,3,4,5].
Due to a lack of paediatric donors, technical-variant grafts are often used despite an associated increase in the rate of surgical complications [6].
Both biliary strictures and bile leaks are the most frequent early post-transplantation complications [4, 7], reportedly occurring within a range of 10–30%. Risk factors for the development of biliary complications include partial liver graft, liver graft cold ischaemia times, impaired graft arterial supply, rejection, and cytomegalovirus (CMV) infection [4, 5, 8,9,10,11,12]. Although these complications do not usually result in graft loss, they are undoubtedly a major cause of significant morbidity in liver graft recipients.
Vascular complications represent a second group of severe complications after liver transplantation [13]. Despite their lower incidence, they can lead to graft loss as well as recipient death [14,15,16]. Risk factors in their development include technical errors during suturing of small-calibre vessels, graft rejection, long liver graft cold ischaemia times, cardiovascular instability, infections in the graft vicinity, oppression by surrounding structures, and fluid collection. Bleeding, stenosis, thrombosis, and aneurysms can occur at any stage during vascular anastomosis, with incidence of up to 25% in paediatric transplantation procedures. Incidence of the most frequent complication, hepatic artery (HA) stenosis/thrombosis, is reported at 10–20%. Since HA thrombosis during the early postoperative period can result not only in graft loss but also jeopardise the life of the recipient, aggressive management as an emergency procedure is recommended [3]. While the incidence of portal vein (PV) stenosis is 4–8%, hepatic vein (HV) and inferior vena cava (IVC) stenoses reportedly occur in less than 2% of graft recipients [5, 17,18,19].
The aim of this study, therefore, was to assess the efficacy of percutaneous interventional techniques in the management of vascular and non-vascular complications following liver transplantation in paediatric patients.
Material and Methods
During the 2001–2018 period, a total of 105 paediatric liver transplantation procedures were performed at our centre, with all grafts obtained from cadaveric donors. Using percutaneous interventional procedures, we successfully managed 25 liver transplantation-related complications in 23 children (22.3%) (Tables 1 and 2).
Transplantation Technique
According to paediatric transplantation protocol, we use split or reduced liver grafts (usually segments II and III) based on the results of CT volumetry. To flush the liver graft, in the majority of cases we use the protective solution Custodiol® HTK (Dr. Franz Kohler Chemie GmbH, Bensheim, Germany) and, less frequently (5 cases in this study), ViaSpan® (University of Wisconsin [UW] solution, DuPont Pharmaceuticals, Wilmington, DE, USA).
Once the PV is sutured end to end, liver perfusion is resumed followed by HA anastomosis, with bile ducts the last tubes to be reconstructed. In cases where the common bile duct is long enough and the underlying liver disease permits, a simple end-to-end anastomosis to the recipient bile duct is created. In all other cases, the original reconstructed Roux-Y loop is used, or created and sutured to a hepaticojejunal (HJ) anastomosis. The anastomosis can be subsequently secured with a temporary stent.
Immunosuppressive Protocol
Our protocol is a combination of tacrolimus and corticosteroids, with the addition of mycophenolate mofetil in children aged over 15 years. In patients undergoing liver retransplantation, the monoclonal antibody basiliximab is used for induction.
Postoperative Follow-Up
Parameters such as immunosuppression levels are monitored during the postoperative follow-up period by liver tests and Doppler ultrasound. Percutaneous liver graft biopsies are performed in suspected cases of graft rejection.
Biliary Complications (Table 1).
Biliary complications occurred in 14 patients (7 males, 7 females) (13.3%), representing a mean age of 5.9 (0.4–15.2) years. The average interval between liver transplantation and intervention was 203.9 (10–600) days. Eleven patients underwent primary transplantation, 2 underwent retransplantation, and 1 required a third transplantation procedure. Two patients received full-size grafts, 4 patients received reduced-size grafts, and 8 patients received split grafts. We intervened in 10 cases of HJ anastomosis, 3 cases of biHJ anastomosis, and 1 case of choledocho-choledochal anastomosis. Procedure indications were anastomotic biliary leaks in 3 cases and a surface leak in 1. Ten cases involved bile duct obstruction followed by cholestasis, combined with cholangitis in 5 cases, of which 1 was abscessed (Table 1). External–internal drainage was attempted in all patients after bile duct visualisation by ultrasound and magnetic resonance cholangiopancreatography (MRCP) in the majority of patients.
Biliary intervention was performed under general anaesthesia during both initial and repeat procedures, with sedation only used for simple procedures such as catheter exchange. Biliary drainage was initiated using a 21G puncture needle (21G Trocar Needle, Cook Medical, Denmark, or AccuStick II, Boston Scientific, USA) and in most cases with the help of ultrasound navigation. To negotiate biliary stenosis or obstruction, we used the Selectiva guidewire (Heraeus, USA) and, where necessary, an 0.018-inch infrapopliteal guidewire (V-18 Control Wire, Boston Scientific, USA). Balloon dilatation was performed using small profile balloons originally designed for infrapopliteal angioplasty compatible with a 0.018-inch guidewire (Sterling PTA Balloon, Boston Scientific, USA; Savvy, Cordis, Ireland) in combination with a 4-5F sheath (AccuStick II sheath in most cases). Diameters of the dilatation balloons were in the range of 3.0–4.5 mm based on the bile duct size dilated. After dilatation, a 6F biliary catheter with locking pigtail (Neo-Soft biliary drainage catheter, BioTeq, Germany) was left in the dilated duct for 6–8 weeks for external–internal drainage. In cases where we failed to cross the stenosis or obstruction, a 6F pigtail drainage catheter was used. After considering the size and weight of the child, in cases where leak healing required a longer period of drainage or repeat dilatation, we exchanged the 6F catheter for an 8F size (Navarre biliary drainage catheter, Bard, USA). Six to eight weeks after dilatation, we performed follow-up cholangiography. In cases of persisting stenosis or restenosis, we continued dilatation using a balloon of the same size or 0.5 mm larger, an approach repeated anywhere up to three times. In cases where the dilated bile duct was not patent or the leak persisted, the percutaneous technique was considered to have failed, with the patient either referred for corrective surgery (including retransplantation) or permanent drainage continued.
Vascular Complications (Table 2).
Overall, vascular percutaneous interventions were performed in 11 patients (7 males, 4 females) (10.5%), representing a mean age of 10.1 (1–17.1) years. The average interval between transplantation and intervention was 40.5 (13–150) days. Primary, secondary, and tertiary transplantations were performed in 5, 4, and 2 cases, respectively. Two patients received full-size grafts, 5 received reduced-size grafts, and 4 received split grafts. All interventional procedures were performed under general anaesthesia, except in the cases of 3 female patients close to young adolescence where sedation was tolerated.
The interventions involved HA in 5 cases (HA stenosis in 3 and occlusion in 2). The clinical reason for intervention in all five cases was deteriorating liver function. All 5 HA PTA/stenting procedures were performed using the femoral artery approach, with the Micropuncture Introducer Set (Cook Medical, Denmark) used for the initial puncture. After successful crossing of the stenosis/occlusion either using the 0.014-inch coronary (Pilot, Abbot, USA) or 0.018-inch peripheral (V-18 Control Wire, Boston Scientific, USA) guidewire, the lesion site was predilated using either the Tazuna balloon (Terumo, USA) for the 0.014-inch guidewire or the Savvy balloon (Cordis, USA) for the 0.018-inch guidewire. In two patients, 7-mm/4-cm (Wallstent, Boston Scientific, USA) and 6-mm/4-cm (X-pert, Abbot, USA) bare metal stents were placed using the 0.018-inch wire. A drug-eluting 4-mm/4-cm coronary stent (Synergy, Boston Scientific, USA) was placed in the predilated segment in another patient. In 1 patient only, dilatation was performed using a 2.5-mm Tazuna balloon after unsuccessful attempt to place a stent to HA.
Two patients with lower limb oedema and ascites underwent stenotic IVC stenting using the femoral vein approach. In both cases, we placed the Sinus XL stent (Optimed, Germany) dilated to 16 mm using the Zelos (Optimed, Germany) 16-mm/4-cm dilatation balloon.
One case required stenting of a stenotic PV was based on indications of portal hypertension and oesophageal varices. After predilatation with a 4-mm balloon (Savvy, Cordis, USA), a 6-mm/4-cm stent (X-pert, Abbot, USA) was placed using a transhepatic PV approach.
Two patients underwent embolisation of branches of the superior mesenteric artery (SMA) due to gastrointestinal (GI) bleeding. In 1 case, embolisation was performed after an iatrogenic arteriobiliary fistula developed during biliary intervention (Tables 1 and 2). All embolisations were performed using the femoral artery approach and a micropuncture set. Arteries and branches for occlusion were embolised using 0.018-inch coils (Cook Medical, Denmark).
Results
Biliary Interventions
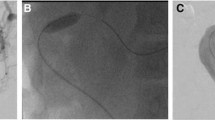
In 12 out of 14 cases (85.7%), we inserted an external–internal biliary drain, supplemented with balloon dilatation of mostly anastomotic strictures. In 7 cases, the external–internal biliary drain was removed after 4–8 weeks. Despite successful external–internal biliary drainage, biliary leaks persisted in 2 patients presenting with a combination of leaks and strictures: one underwent retransplantation and the other HJ anastomosis resuturing. One patient who had external–internal biliary drainage of only one of two obstructed bile ducts experienced persistent cholangitis, requiring urgent retransplantation. Another 2 patients developed recurrent cholestasis requiring the insertion of an external–internal biliary drain, with one of these patients reassigned to the waiting list for retransplantation (Fig. 1). The other patient was given balloon dilatation and external–internal drainage. Although the drainage catheter was removed after eight weeks, the stenosis persisted. Therefore, further dilatation and subsequent external–internal drainage were performed. We removed the drainage catheter after 8 weeks (at which point the stenosis persisted) before reinserting the drainage catheters; the patient was later reassigned to the waiting list for retransplantation.
Split graft.A: Total occlusion of anastomosis and dilatation of bile ducts. B: Anastomosis crossed from the lower duct. C: Balloon dilatation of the middle duct from the lower duct, leaving only one external–internal drain. D: Anastomotic dilatation from the lower duct. E: External–internal drainage catheter introduced through the lower duct. F: Persistent cholangitis; middle duct occluded and moderately dilated (arrow). G, H: Another drain inserted from the middle duct to establish long-term double external–internal drainage; cholangitis controlled and liver function within normal limits
In 2 out of 14 (14.3%) cases, we failed to cross the anastomotic occlusion; these interventions consisted of external drainage only, with both patients reassigned to retransplantation on a further date. One of them was a patient in whom HA reoccluded three months after PTA recanalisation. While establishing biliary drainage in one patient, we encountered a potentially serious complication involving the formation of an arteriobiliary fistula leading to substantial haemobilia; the fistula was eliminated by embolisation (Tables 1 and 2).
Vascular Interventions
Of the 5 patients requiring HA interventions, 4 underwent successful HA recanalisation by angioplasty (a stent was placed in 3 of them) leading to subsequent improvement/normalisation in liver function. The patient with unsuccessful HA stent placement where PTA only was performed reoccluded 4 months later without worsening of major liver function. This patient developed biliary anastomotic occlusion 8 months later, at which point we applied external biliary drainage; the patient was later retransplanted (patient 10 in Table 1 and patient 8 in Table 2). We failed to cross a HA occlusion in one case, with the patient undergoing urgent retransplantation. Irrespective of site, all three cases of embolisation—involving GI bleeding from SMA branches in two patients and arteriobiliary fistula formation in one patient—were successful both technically and clinically. IVC and PV stenoses in two patients were successfully dilated and stented. None of the vascular procedures resulted in complications.
Discussion
The predominant complications in our group of paediatric patients indicated for liver transplantation were biliary stenosis or leakage. Based on our results, partial graft transplantation was the procedure associated with the highest risk of developing biliary and vascular complications after liver transplantation. This procedure involves the complex arrangement of anatomical structures, which can result in bile duct ischaemia, particularly in the resection area close to the bile duct. We consider this the underlying cause of most biliary complications in our group, prompting us to rule out other possible causes such as rejection, older-age liver donors, and CMV infection. Interestingly, the incidence of biliary complications we observed—both leaks and obstructions—was relatively low compared to other incidences reported in the literature (13.3% in our group compared to 10–30% in other series) [4, 5, 7, 12].
Biliary Lesions (Table 1).
Although percutaneous interventions such as dilatation and stenting are now recognised methods in the management of biliary strictures, the data currently used to identify factors affecting outcomes are inconsistent [20]. Factors such as chronic rejection, graft type, CMV infection, and underlying disease do not seem to impact on the outcomes of percutaneous interventions [20, 21]. A higher incidence of stenosis has been reported for duct-to-duct anastomosis [5], despite some authors disregarding the association [6, 22,23,24]. Most studies suggest that stenosis of HJA to the Y-Roux loop and, in particular, non-anastomotic stenoses are frequently associated with HA stenosis/HA thrombosis (HAS/HAT) [25]. In our patients, the incidence of HAS/HAT did not correlate closely with that of biliary stenosis or leakage, most likely because HA stenosis/occlusion was endovascularly removed early in 4 out of 5 patients. In 1 patient, biliary stricture developed eight months after reocclusion of HA (the only HA not stented); this individual was kept on external biliary drainage before undergoing subsequent retransplantation.
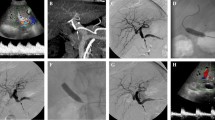
An anastomotic biliary leak (Fig. 2) can be caused by surgical error typically associated with duct-to-duct anastomosis [4] or, like strictures, by ischaemia in HAS/HAT or PV stenosis/PV thrombosis. A somewhat infrequent complication is leakage from the cut surface of a split or reduced-size graft [26] (Fig. 3). This can manifest as an admixture of bile from surgical drainage, in turn leading to perihepatic fluid collection, biliary peritonitis, fever, or leucocytosis. A more detailed view of the leak site can be obtained by MRI cholangiography.
The only complication arising from biliary intervention we encountered was an arteriobiliary fistula, managed successfully by superselective embolisation without any sequelae.
Vascular Lesions (Table 2).
Vascular complications occurring in connection with paediatric liver transplantation typically require intervention and particularly apply to HAT and HAS when diagnosed in the early post-transplant period (within 4 weeks) [15]. A diagnosis is usually established based on the results of Doppler ultrasound with confirmation by computer tomography angiography (CTA). Because early HAT can lead to bile duct ischaemia/necrosis and subsequent sepsis, urgent intervention is mandatory [27]. Where HAS/HAT develops at a later post-transplant stage, the clinical course can prove less dramatic, occasionally confined solely to elevated transaminase levels; however, in some cases, ischaemic bile duct stenosis is a potential complication [15]. While urgent retransplantation has been long considered the method of choice, endovascular approaches are currently being used as a first-line treatment in a number of centres. These strategies involve PTA, frequently complemented with stent placement (Fig. 4) and/or, on occasion, intra-arterial thrombolysis [2, 3, 16, 28]. Given patients with HAS are at increased risk of developing HAT, preventive endovascular intervention is recommended [14]. The problem is that endovascular interventions are, inherently, relatively higher-risk procedures, potentially resulting in thrombosis (in the presence of HAS), rethrombosis, dissection of an artery, and (in rare cases) arterial rupture [19]. The incidence of HAS/HAT in our group of patients was 4.8%, a figure far below the percentages reported in the literature (10–20%) [3, 13, 14].
IVC stenosis in our group included two females close to young adolescence (14.6 and 15.5 years). IVC size is therefore not thought to increase appreciably, with the likelihood that the stents inserted do not limit blood flow through the IVC.
There was one case of PV stent placement involving a 1-year-old child. And although the procedure did help to eliminate oesophageal varices, it remains to be seen whether the diameter of the stent will be large enough to maintain PV patency as the child grows with age. An arterioportal fistula, an accidental finding during HA dilatation, was likewise managed using coil embolisation. In the case of an arteriobiliary fistula, which formed as an iatrogenic lesion after establishing biliary drainage, it was again successfully closed with coils. In two cases complicated by GI bleeding, it was difficult to prove a causal relationship with liver transplantation. We included both of these cases in our series, given the time since transplantation was relatively short. Both were treated successfully by coil embolisation.
Conclusions
Percutaneous interventions are considered safe and efficient for treating paediatric liver transplantation complications, both biliary and vascular. They are recommended as first-line treatments for such complications, but may need to be supplemented with surgery. Not only can they be used as a bridge to surgery, percutaneous techniques can also aid surgery planning while reducing the need for urgent retransplantations.
Change history
04 March 2020
In the original article, the following author name was incorrectly published and the corrected name is given below.
References
Bucuvalas J. Long-term outcomes in pediatric liver transplantation. Liver Transpl. 2009;15(Suppl 2):S6–11.
Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9(4):746–57.
Duffy JP, Hong JC, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, Busuttil RW. Vascular complications of orthotopic liver transplantation: experience in more than 4200 patients. J Am Coll Surg. 2009;208(5):896–903 discussion 903–5.
Karakayalı F, Kırnap M, Akdur A, Tutar N, Boyvat F, Moray G, Haberal M. Biliary complications after pediatric liver transplantation. Transplant Proc. 2013;45(10):3524–7.
Laurence JM, Sapisochin G, DeAngelis M, Seal JB, Miserachs MM, Marquez M, Zair M, Fecteau A, Jones N, Hrycko A, Avitzur Y, Ling SC, Ng V, Cattral M, Grant D, Kamath BM, Ghanekar A. Biliary complications in pediatric liver transplantation: Incidence and management over a decade. Liver Transpl. 2015;21(8):1082–90.
Diamond IR, Fecteau A, Millis JM, Losanoff JE, Ng V, Anand R, Song C, SPLIT Research Group. Impact of graft type on outcome in pediatric liver transplantation: a report from Studies of Pediatric Liver Transplantation (SPLIT). Ann Surg. 2007;246(2):301-10.
Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13(2):253–65.
Wojcicki M, Silva MA, Jethwa P, Gunson B, Bramhall SR, Mayer D, Buckels JA, Mirza DF. Biliary complications following adult right lobe ex vivo split liver transplantation. Liver Transpl. 2006;12(5):839–44.
Keogan MT, McDermott VG, Price SK, Low VH, Baillie J. The role of imaging in the diagnosis and management of biliary complications after liver transplantation. AJR Am J Roentgenol. 1999;173(1):215–9.
Wang SF, Huang ZY, Chen XP. Biliary complications after living donor liver transplantation. Liver Transpl. 2011;17(10):1127–36.
Vagefi PA, Parekh J, Ascher NL, Roberts JP, Freise CE. Outcomes with split liver transplantation in 106 recipients: The University of California, San Francisco, experience from 1993 to 2010. Arch Surg. 2011;146(9):1052–9.
Darius T, Rivera J, Fusaro F, Lai Q, de Magnée C, Bourdeaux C, Janssen M, Clapuyt P, Reding R. Risk factors and surgical management of anastomotic biliary complications after pediatric liver transplantation. Liver Transpl. 2014;20(8):893–903.
Kamran S, Mirzakhani H, Eslami M, Saidi RF. Current state of the art in management of vascular complications after pediatric liver transplantation. Pediatr Transplant. 2015;19(1):18–26.
Sieders E, Peeters PM, TenVergert EM, de Jong KP, Porte RJ, Zwaveling JH, Bijleveld CM, Slooff MJ. Early vascular complications after pediatric liver transplantation. Liver Transpl. 2000;6(3):326–32.
Porrett PM, Hsu J, Shaked A. Late surgical complications following liver transplantation. Liver Transpl. 2009;15(Suppl 2):S12–S1818.
Carnevale FC, de Tarso MA, Moreira AM, Dos Santos AC, da MottaLealFilho JM, Suzuki L, Cerri GG, Tannuri U. Long-term results of the percutaneous transhepatic venoplasty of portal vein stenoses after pediatric liver transplantation. Pediatr Transplant. 2011;15(5):476–81.
Uller W, Knoppke B, Schreyer AG, Heiss P, Schlitt HJ, Melter M, Stroszczynski C, Zorger N, Wohlgemuth WA. Interventional radiological treatment of perihepatic vascular stenosis or occlusion in pediatric patients after liver transplantation. Cardiovasc Intervent Radiol. 2013;36(6):1562–71.
Buell JF, Funaki B, Cronin DC, Yoshida A, Perlman MK, Lorenz J, Kelly S, Brady L, Leef JA, Millis JM. Long-term venous complications after full-size and segmental pediatric liver transplantation. Ann Surg. 2002;236(5):658–66.
Abad J, Hidalgo EG, Cantarero JM, Parga G, Fernandez R, Gomez M, Colina F, Moreno E. Hepatic artery anastomotic stenosis after transplantation: treatment with percutaneous transluminal angioplasty. Radiology. 1989;171(3):661–2.
Sunku B, Salvalaggio PR, Donaldson JS, Rigsby CK, Neighbors K, Superina RA, Alonso EM. Outcomes and risk factors for failure of radiologic treatment of biliary strictures in pediatric liver transplantation recipients. Liver Transpl. 2006;12(5):821–6.
Moreira AM, Carnevale FC, Tannuri U, Suzuki L, Gibelli N, Maksoud JG, Cerri GG. Long-term results of percutaneous bilioenteric anastomotic stricture treatment in liver-transplanted children. Cardiovasc Intervent Radiol. 2010;33(1):90–6.
Chok KS, Chan SC, Chan KL, Sharr WW, Tam PK, Fan ST, Lo CM. Bile duct anastomotic stricture after pediatric living donor liver transplantation. J Pediatr Surg. 2012;47(7):1399–403.
Haberal M, Karakayali H, Atiq A, Sevmis S, Moray G, Ozcay F, Boyvat F. Duct-to-duct biliary reconstruction without a stent in pediatric living-donor liver transplantation. Transplant Proc. 2011;43(2):595–7.
Feier FH, Seda-Neto J, da Fonseca EA, Candido HL, Pugliese RS, Neiva R, Benavides MR, Chapchap P. Analysis of factors associated with biliary complications in children after liver transplantation. Transplantation. 2016;100(9):1944–54.
Sawyer RG, Punch JD. Incidence and management of biliary complications after 291 liver transplants following the introduction of transcystic stenting. Transplantation. 1998;66(9):1201–7.
Righi D, Franchello A, Ricchiuti A, Breatta AD, Versace K, Calvo A, Romagnoli R, Fonio P, Gandini G, Salizzoni M. Safety and efficacy of the percutaneous treatment of bile leaks in hepaticojejunostomy or split-liver transplantation without dilatation of the biliary tree. Liver Transpl. 2008;14(5):611–5.
Singhal A, Stokes K, Sebastian A, Wright HI, Kohli V. Endovascular treatment of hepatic artery thrombosis following liver transplantation. Transpl Int. 2010;23(3):245–56.
PérezSaborido B, PachecoSánchez D, BarreraRebollo A, AsensioDíaz E, PintoFuentes P, SarmenteroPrieto JC, RodríguezVielba P, MartínezDíaz R, GonzaloMartín M, Rodríguez M, CaleroAguilar H, PintadoGarrido R, GarcíaPajares F, AntaRomán A. Incidence, management, and results of vascular complications after liver transplantation. Transplant Proc. 2011;43(3):749–50.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
For this type of study, formal consent is not required.
Informed consent
For this type of study, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peregrin, J.H., Kováč, J., Prchlík, M. et al. Interventional Radiological Treatment of Paediatric Liver Transplantation Complications. Cardiovasc Intervent Radiol 43, 765–774 (2020). https://doi.org/10.1007/s00270-020-02430-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-020-02430-8