Abstract
Purpose
To evaluate long-term arterial patency and abnormalities of bile ducts in patients that had endovascular treatment for arterial complications after liver transplantation (LT).
Materials and Methods
Between 2004 and 2014, 1048 LTs were consecutively performed in our institution and 53 patients (42 men; age range 19–69) were diagnosed and treated by endovascular techniques for arterial complications such as stenosis, thrombosis, dissection or kinking of the hepatic artery (HA). Radiological and surgical data were retrospectively analyzed, and survivors were contacted to undergo follow-up Doppler ultrasound (DUS) of the HA and magnetic resonance cholangiopancreatography.
Results
The primary technical success of endovascular treatment was 94% (n = 50). The patency rate of HA at 5-year was 81%. After a median follow-up of 58 months, 17 patients (32%) developed radiological features of ischemic cholangiopathy (IC), including 7 patients with abnormal DUS and 10 with normal DUS. Patients who presented with complications of the HA in the first 3 months after LT developed IC more frequently (42%) than others (12%) (p = 0.028). No other factor was associated with the development of IC.
Conclusion
IC was more often observed when HA complication occurred within the first 3 months after LT. The presence of IC was not excluded by a normal DUS during follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Implications for Patient Care
-
1.
The only risk factor of ischemic cholangiopathy development after liver transplantation is the early occurrence of arterial complication even if the endovascular treatment was initially successful.
-
2.
Ischemic cholangiopathy cannot be excluded by a normal Doppler ultrasound exam during follow-up after liver transplantation.
-
3.
In patients with abnormal Doppler ultrasound during follow-up after liver transplantation, only the half developed ischemic cholangiopathy.
Summary Statement
If the arterial complication occurred within the first three months after transplantation, the risk of ischemic cholangiopathy is 42%, whatever the presence or not of normal Doppler ultrasound.
Introduction
Biliary complications are important causes of morbidity and mortality after liver transplantation (LT), with an incidence of 10–30% [1,2,3,4]. Ischemic cholangiopathy (IC) can severely damage the biliary tree and the management of these lesions can be difficult, often extending over many years or requiring re-transplantation. Hepatic artery (HA) thrombosis represents a major cause of IC [3, 5]. Risk factors for the development of HA thrombosis such as anastomotic stenosis [6] are actively searched for by Doppler ultrasound (DUS), immediately after transplantation as well as during the lifetime of transplanted patients. Multidetector-computed tomography arteriography (MDCTA) is performed to confirm the diagnosis when stenosis or thrombosis is suspected on DUS and endovascular treatments, such as percutaneous angioplasty (PTA) or stenting are performed [7]. These treatments have been shown to effectively recanalize the artery with lower morbidity and mortality than open surgery [8, 9]. Although these techniques demonstrated to be safe and efficient [8,9,10,11,12,13,14,15,16], the long-term patency of HA, as well as the delayed consequences on the biliary tree, is still a subject of debate [2, 5, 17,18,19,20,21]. Furthermore, because not all patients with HA thrombosis or stenosis develop IC, the identification of protective or predisposing factors is also important.
The aim of this study was to evaluate the long-term outcome of endovascular treatment based on arterial patency by DUS, as well as to evaluate bile duct damage on MRCP.
Materials and Methods
The institutional review board approved this study and informed consent was waived. All patients who underwent endovascular treatment for HA complication after LT between January 2004 and December 2014 were extracted from the picture archiving and communication system (PACS) of our hospital. Clinical, surgical, imaging, laboratory and follow-up data were retrospectively evaluated. Surgical procedures used standard techniques for orthotopic LT or split grafts. Patients presenting with an arterial complication during surgery were immediately treated surgically and were therefore not included in this study.
Diagnosis of HA Complications
In our institution, the diagnosis of HA thrombosis and stenosis was based on a two-step algorithm. Systematic postoperative DUS of the liver graft vasculature was performed daily for the first 5 days, then weekly for 1 month and every 6 months thereafter. Arterial complications were suggested on DUS in the presence of one or more of the following features: (1) post-anastomotic HA resistive index < 0.5; (2) time to peak > 0.08 s); (3) tardus-parvus waveform distal to the stenosis; (4) increased peak systolic velocity (> 200 cm/s) at the stenosis [22] (Fig. 1A). If a complication was suspected, patients rapidly underwent MDCTA (Fig. 1B).
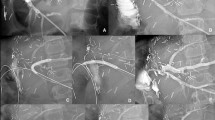
Endovascular treatment of an anastomotic stenosis of the hepatic artery in a 62-year-old transplanted patient. A Doppler-US of the donor hepatic artery showing a tardus-parvus waveform (resistive index, 0.39; time to peak, 0.22 s). B Coronal MIP CT image showing a short anastomotic stenosis (arrow). C Hepatic angiogram confirming a short stenosis > 70%. D Angioplasty with a 5-mm balloon. E Control angiogram showing a residual stenosis > 50%. F Placement of a balloon-expandable stent (diameter, 5 mm; length, 15 mm). G Control angiogram showing absence of residual stenosis. H Control Doppler-US showing a normal waveform (resistive index, 0.64; time to peak, 0.06 s)
During the study period, MDCT examinations were performed with a bolus tracking technique in the arterial phase and with a 70 s delay in the portal phase with the following parameters: intravenous administration of a 2 mL/kg of non-ionic contrast material (350 mg iodine); injection rate, 4 mL/s with a mechanical power injector; reconstruction slice thickness, 1.25 mm. If the diagnosis of HA thrombosis or stenosis was confirmed, patients were referred to the department of interventional radiology for treatment (Fig. 1C).
Endovascular Techniques
Endovascular treatment included percutaneous transluminal angioplasty (PTA) with or without bailout stenting performed under local anesthesia. In the presence of existing thrombosis of the HA or if thrombosis was discovered during treatment, catheter-directed thrombolysis (CDT) was performed. We favored femoral access and 6-French introducer sheaths to guide catheters. We mainly used low-profile balloons, short self-expanding nitinol stents and balloon-expandable metal stents (Fig. 1C–E). Balloon and stent size ranged from 4 to 5 mm in diameter and 12–20 mm in length. Stenting was only performed in the presence of residual stenosis or a flow-limiting dissection, and covered stent was used in case of an associated pseudoaneurysm [8, 15] (Fig. 1F, G). CDT was performed with alteplase (Actilyse; Boehringer Ingelheim), in case of acute arterial thrombosis. A bolus of 8 mg was injected manually in the main thrombus via a 4-F multisided hole-dedicated catheter (Fountain infusion system; Merit Medical Systems), followed by a slow infusion of 1 mg/h for 12 h. A second angiogram was performed at the end of the infusion to confirm recanalization. All patients were given anticoagulation therapy during the procedure including an intra-arterial bolus dose of 45 IU/kg of unfractionated heparin. After the procedure, patients received 100 mg of acid acetylsalicylic per day for life. Clopidogrel 75 mg/day was added for three months if a stent was placed.
Follow-Up Surveillance
Patients were followed up until death, lost to follow-up or last follow-up. The study ended on October 2016. HA patency was assessed by DUS every 6 months with the same criteria as for postoperative DUS (Fig. 1H). Early and late restenosis were defined as recurrent HA stenosis less or more than 1 month after angioplasty, respectively [10]. MR imaging at 1.5-T, including MRCP, was performed either yearly for LT follow-up, or for investigation of biliary complications suspected on clinical and biological findings. The protocol for MRCP included radial 2D single shot breath-hold and 3D turbo spin echo T2-weighted sequences. IC was defined as the development of biliary anomalies, excluding biliary anastomotic strictures and recurrent sclerosing cholangitis, such as (1) extra- or intrahepatic ductal strictures; (2) extra- or intrahepatic ductal dilatations; (3) intrahepatic biloma; (4) complete biliary destruction, with irregular dilatation of the entire biliary tree and presence of biliary casts [23,24,25] (Fig. 2A–F). To confirm that patients were properly screened and reduce a possible observation bias, all survivors were recalled to undergo both DUS and MRCP, the former to assess HA patency, and the latter to evaluate the presence of biliary anomalies. When patients died or were lost to follow-up before the end of the study, we analyzed the last DUS and MRCP retrieved from the PACS. A consensus analysis of images was obtained from two abdominal radiologists with 10 and 15 years of experience (blinded).
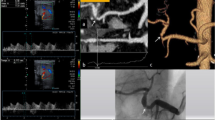
Ischemic cholangiopathy following occlusion of the hepatic artery 8 months after liver transplantation in a 52-year-old patient. A Coronal MIP CT image showing an occlusion of the hepatic artery (arrows) and an intrahepatic biloma (asterisk). B MR cholangiogram showing an irregular dilatation of the donor biliary tract, extending to the anastomosis and sparing recipient biliary main duct (arrow). C 3D-MRCP image showing the abnormal bile ducts (black arrowheads), surrounded by periportal fluid (white arrows) communicating with the biloma (asterisk). D Hepatic angiogram confirming a complete occlusion of the hepatic artery (arrows). E Control angiogram after catheter-directed thrombolysis and placement of a nitinol stent (diameter, 4 mm; length, 20 mm) showing patency of the hepatic artery. F Follow-up MR cholangiogram 3 weeks after endovascular treatment showing progression of the biliary destruction with casts formation (arrows)
Data Analysis
Results are presented as mean ± standard deviation or median and range for quantitative data, and as the number of cases (percentage of cases) for categorical variables. Comparisons were performed with the Student-t test and the Mann–Whitney U test for continuous variables. Qualitative data were compared with the Chi-square or Fisher’s exact tests when necessary. Patient characteristics including demographic, surgical, interventional, clinical and laboratory data associated with the occurrence of IC were identified. Survival time corresponded to the delay between the date of the endovascular procedure and death, the end of the study period or the last visit. Survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. Variables were analyzed by the receiver-operating characteristic (ROC) to determine the optimal cut-off time for post-transplant arterial complications, and Kaplan–Meier curves were reconstructed using this cut-off value. Tests were always two-sided, and p < 0.05 was considered significant. All analyses were performed using Statistical Package for the Social Sciences (SPSS) software (version 20.0, IBM SPSS Inc).
Results
Patients’ Characteristics
Fifty-three consecutive patients (mean age, 50 ± 12 years; 42 males) were included in this study and represented 5.05% of the transplanted population (N = 1048) in our institution during the study period. Forty-one (77%) transplantations were orthotopic LT, and 12 (23%) were split grafts. Forty-seven (88%) patients had bilio-biliary anastomosis and 6 (12%) had bilio-enteric anastomoses. Most arterial anastomoses were end-to-end between the HA of the recipient and the donor (n = 40, 83%). At transplantation, the MELD score was 14.0 ± 7.6, the age of liver donor was 55 ± 17 years, and the cold/warm ischemic time was 502 ± 158 min, and 50 ± 15 min, respectively.
HA Complications and Endovascular Treatments
The indications for primary endovascular treatment are described in Table 1. The initial endovascular procedures in the 53 patients were PTA in 18 (34%), PTA with bailout stenting in 32 (60%) and PTA with or without stenting and CDT in 3 (6%). The primary technical success rate was 94% (n = 50). The technical failures were impossibility to cross a non-anastomotic stenosis (n = 1), arterial tortuosity (n = 1), and median arcuate ligament with associated stenosis (n = 1). Treatment was attempted again in all three patients with one secondary success (arterial tortuosity) and two failures. Six (11%) adverse events were recorded, including one post-procedural major complication (a pseudoaneurysm detected and treated by a covered stent the following day), and five peri-procedural minor complications (four acute HA thromboses immediately treated by CDT and one HA dissection treated by stenting).
Forty-two (79%) patients underwent one endovascular procedure, nine had two procedures (17%), one had three procedures (2%) and one had four procedures (2%), for a total of 14 re-interventions. Most re-interventions were performed for restenosis of the HA (N = 10). The patency rate of HA at 1-month, 6-month, 1-year, 3-year and 5-year, was 89, 87, 83, 81 and 81%, respectively.
Follow-Up and Factors Associated with Ischemic Cholangiopathy
The mean follow-up for the entire cohort was 58 months (16–124). The median delay between surgery and the diagnosis of arterial complications was 5.4 months (0.1–63.6), and the median delay between diagnosis and endovascular treatment was 13 days (1–90). Thirty-six patients (68%) were recalled for DUS and MRCP. Thirteen (25%) of the remaining patients died, two were lost to follow-up and two refused to undergo MRCP. The causes of death were recurrent hepatocellular carcinoma (n = 6), postoperative complications after re-transplantation (n = 2), severe acute pancreatitis (N = 1), IC (n = 1), multiple organ failure (n = 1), lymphoma (n = 1) and chronic rejection (n = 1).
DUS examinations were normal according to the defined criteria in 38 (72%) patients and abnormal in the remaining 15 (28%) patients. Features of IC were found in 17 (32%) patients at MRCP, and the remaining 36 (68%) patients had no biliary abnormalities. The two patients who did not undergo MRCP had no symptoms, normal liver tests and absence of biliary abnormalities at MDCT. Ten of the 36 (26%) patients with normal DUS on follow-up developed signs of IC, and 28 (74%) did not, whereas seven of the 15 patients (47%) with abnormal follow-up DUS developed features of IC, and 8 (53%) did not (p = 0.197). Patients with IC had a shorter median survival than those without (77 months (95% CI 53–101) versus 133 months (95% CI 117–148), p = 0.0163) (Fig. 3). A cut-off value of 3 months between LT and arterial complications identified two groups of patients, with a significantly different risk of developing IC (42% [n = 15] vs. 12% [n = 2], for complications < 3 and > 3 months, respectively, p = 0.028) (Table 2). No other factors were shown to be associated with the development of IC.
Discussion
This study confirmed the feasibility and safety, as well as the good long-term outcome of endovascular treatment for HA complications secondary to LT, with 72% of patients showing normal DUS results of the HA. These results are similar to other studies showing patency rates of between 77 and 79% [9, 10, 12, 13, 22]. Nevertheless, around one-third of our patients developed IC, which is also consistent with previous reports [2, 20, 21, 26]. The only factor associated with the occurrence of IC identified in this study was the delay between LT and the onset of arterial complications. These results suggest that patients with arterial complications related to LT, especially within 3 months after surgery, had a greater risk of developing IC. Interestingly, no other factors were found to be associated with the development of IC. Technical factors, including initial technical success, peri- and post-procedural complications or the recurrence of arterial anomalies were not associated with a higher risk of developing IC. If the arterial complication occurred within the first three months after transplantation, the risk of IC is 42%, whatever the presence or not of Doppler abnormalities.
In 2015, Pulitano et al. [21] published a study comparing biliary complication-free survival of patients treated, or not, for HA stenosis after LT. The authors showed that endovascular treatment of the HA was beneficial if the stenosis developed within 6 months after LT. They also estimated that patients were unlikely to develop biliary complications after this critical period and thus would not benefit from recanalization [21]. The present results partially support these findings and thus sustain the hypothesis that patients may develop arterial collateral vessels over time. Interestingly, long-term follow-up showed that one-third of patients who developed IC had normal DUS results of HA, and half of the patients with abnormal DUS did not develop IC. This suggests that causes aside from impaired arterial inflow to the peribiliary plexus itself [19] must also be present to develop IC.
Nevertheless, despite prompt endovascular treatment, transplanted patients with vascular complications still develop biliary ischemic lesions more frequently than those without (3–17% in published studies versus 32% in the present study) [17]. Nevertheless, our rate of biliary complications is comparable to that found in similar series (31.4%) [21] and lower than in patients with arterial complications receiving no treatment (67%) [20]. This also supports the necessity of associated causes for the development of IC, such as donor age > 50 years, greater donor body weight, cytomegalovirus co-infection, recurrent sclerosing cholangitis, ABO-incompatible transplantations, graft rejection, prolonged warm ischemic time, and delayed re-arterialization in case of sequential revascularization during LT [2, 3, 22].
It is important to note that even though the survival rate in patients with IC was lower than that in patients who did not develop biliary lesions in our series, the mortality rate of the entire cohort was similar to that in studies of transplanted patients without IC [27, 28]. This is mainly due to the severity of biliary lesions. Indeed, only a few patients had severe biliary disease that threatened graft survival and most had moderate biliary disease that could be treated percutaneously or endoscopically. This may have been due to the beneficial effect of arterial recanalization on the severity of cholangiopathy, although this is beyond the scope of this study.
This study has several limitations. Besides its retrospective design, the number of included patients was small, as well as the sample size of the different subgroups, decreasing the statistical power of analysis. Nevertheless, this is the largest published cohort evaluating endovascular treatment for consecutive HA complications secondary to LT in well-characterized patients. Also, the absence of a control group and the absence of systematic confirmation of DUS abnormalities by MDCT limits the interpretation of the survival curves. Finally, the accuracy of DUS examinations is variable and dependent on the operator and the correction angle, especially with tortuous HA, yielding false positive results.
In conclusion, systematic endovascular management of arterial impairment after LT is feasible and safe. However, approximately one-third of patients develop biliary ischemic lesions. Even though the mechanism of IC is not clearly understood, our results show that patients who develop post-transplantation arterial complications within 3 months after surgery are at greater risk of developing ischemic lesions, and this subgroup would benefit the most from treatment. Finally, normal DUS of the HA during follow-up does not exclude the presence of IC.
Abbreviations
- CDT:
-
Catheter-directed thrombolysis
- CI:
-
Confidence interval
- DUS:
-
Doppler ultrasound
- HA:
-
Hepatic artery
- IC:
-
Ischemic cholangiopathy
- MDCT/A:
-
Multidetector-computed tomography/arteriography
- MRCP:
-
Magnetic resonance cholangiopancreatography
- LT:
-
Liver transplantation
- PTA:
-
Percutaneous transluminal angioplasty
- PACS:
-
Picture archiving and communication system
References
Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245–57.
Kienlein S, Schoening W, Andert A, Kroy D, Neumann UP, Schmeding M. Biliary complications in liver transplantation: impact of anastomotic technique and ischemic time on short- and long-term outcome. World J Transplant. 2015;5:300–9.
Mourad MM, Algarni A, Liossis C, Bramhall SR. Aetiology and risk factors of ischaemic cholangiopathy after liver transplantation. World J Gastroenterol. 2014;20:6159–69.
Cotroneo AR, Di Stasi C, Cina A, De Gaetano AM, Evangelisti R, Paloni F, et al. Stent placement in four patients with hepatic artery stenosis or thrombosis after liver transplantation. J Vasc Interv Radiol. 2002;13:619–23.
Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9:746–57.
Pareja E, Cortes M, Navarro R, Sanjuan F, Lopez R, Mir J. Vascular complications after orthotopic liver transplantation: hepatic artery thrombosis. Transplant Proc. 2010;42:2970–2.
Vignali C, Bargellini I, Cioni R, Petruzzi P, Cicorelli A, Lazzereschi M, et al. Diagnosis and treatment of hepatic artery stenosis after orthotopic liver transplantation. Transplant Proc. 2004;36:2771–3.
Hamby BA, Ramirez DE, Loss GE, Bazan HA, Smith TA, Bluth E, et al. Endovascular treatment of hepatic artery stenosis after liver transplantation. J Vasc Surg. 2013;57:1067–72.
Le L, Terral W, Zea N, Bazan HA, Smith TA, Loss GE, et al. Primary stent placement for hepatic artery stenosis after liver transplantation. J Vasc Surg. 2015;62:704–9.
Chen GH, Wang GY, Yang Y, Li H, Lu MQ, Cai CJ, et al. Single-center experience of therapeutic management of hepatic artery stenosis after orthotopic liver transplantation. Report of 20 cases. Eur Surg Res. 2009;42:21–7.
Kodama Y, Sakuhara Y, Abo D, Shimamura T, Furukawa H, Todo S, et al. Percutaneous transluminal angioplasty for hepatic artery stenosis after living donor liver transplantation. Liver Transpl. 2006;12:465–9.
Lastovickova J, Peregrin J. Percutaneous transluminal angioplasty of hepatic artery stenosis in patients after orthotopic liver transplantation: mid-term results. Cardiovasc Intervent Radiol. 2011;34:1165–71.
Maruzzelli L, Miraglia R, Caruso S, Milazzo M, Mamone G, Gruttadauria S, et al. Percutaneous endovascular treatment of hepatic artery stenosis in adult and pediatric patients after liver transplantation. Cardiovasc Intervent Radiol. 2010;33:1111–9.
Rostambeigi N, Hunter D, Duval S, Chinnakotla S, Golzarian J. Stent placement versus angioplasty for hepatic artery stenosis after liver transplant: a meta-analysis of case series. Eur Radiol. 2013;23:1323–34.
Saad WE, Davies MG, Sahler L, Lee DE, Patel NC, Kitanosono T, et al. Hepatic artery stenosis in liver transplant recipients: primary treatment with percutaneous transluminal angioplasty. J Vasc Interv Radiol. 2005;16:795–805.
Sabri SS, Saad WE, Schmitt TM, Turba UC, Kumer SC, Park AW, et al. Endovascular therapy for hepatic artery stenosis and thrombosis following liver transplantation. Vasc Endovascular Surg. 2011;45:447–52.
Abbasoglu O, Levy MF, Brkic BB, Testa G, Jeyarajah DR, Goldstein RM, et al. Ten years of liver transplantation: an evolving understanding of late graft loss. Transplantation. 1997;64:1801–7.
Duffy JP, Hong JC, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, et al. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg. 2009;208:896–903.
Hsiao CY, Ho CM, Wu YM, Ho MC, Hu RH, Lee PH. Management of early hepatic artery occlusion after liver transplantation with failed rescue. World J Gastroenterol. 2015;21:12729–34.
Orons PD, Sheng R, Zajko AB. Hepatic artery stenosis in liver transplant recipients: prevalence and cholangiographic appearance of associated biliary complications. AJR Am J Roentgenol. 1995;165:1145–9.
Pulitano C, Joseph D, Sandroussi C, Verran D, Strasser SI, Shackel NA, et al. Hepatic artery stenosis after liver transplantation: is endovascular treatment always necessary? Liver Transpl. 2015;21:162–8.
Low G, Crockett AM, Leung K, Walji AH, Patel VH, Shapiro AM, et al. Imaging of vascular complications and their consequences following transplantation in the abdomen. Radiographics. 2013;33:633–52.
Boraschi P, Donati F. Postoperative biliary adverse events following orthotopic liver transplantation: assessment with magnetic resonance cholangiography. World J Gastroenterol. 2014;20:11080–94.
Boraschi P, Donati F, Gigoni R, Urbani L, Femia M, Cossu MC, et al. Ischemic-type biliary lesions in liver transplant recipients: evaluation with magnetic resonance cholangiography. Transplant Proc. 2004;36:2744–7.
de Vries Y, von Meijenfeldt FA, Porte RJ. Post-transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. Biochim Biophys Acta. 2017.
Iida T, Kaido T, Yagi S, Hori T, Uchida Y, Jobara K, et al. Hepatic arterial complications in adult living donor liver transplant recipients: a single-center experience of 673 cases. Clin Transplant. 2014;28:1025–30.
Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, et al. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537–46.
Aranzana EM, Coppini AZ, Ribeiro MA, Massarollo PC, Szutan LA, Ferreira FG. Model for End-Stage Liver Disease, Model for Liver Transplantation Survival and Donor Risk Index as predictive models of survival after liver transplantation in 1006 patients. Clinics (Sao Paulo). 2015;70:413–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Breguet, R., Dondero, F., Pupulim, L. et al. Endovascular Treatment of Arterial Complications After Liver Transplantation: Long-Term Follow-Up Evaluated on Doppler Ultrasound and Magnetic Resonance Cholangiopancreatography. Cardiovasc Intervent Radiol 42, 381–388 (2019). https://doi.org/10.1007/s00270-018-2108-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-2108-8