Abstract
Purpose
Arteriovenous malformations (AVMs) are typically congenital in origin, but acquired types, such as dural arteriovenous fistula (AVF), have been described. This study aimed to describe the diagnosis and endovascular treatment of acquired hepatic arterial–portal venous (HA-PV) malformations.
Materials and Methods
A retrospective review of suspected acquired HA-PV malformations from 9/2011 to 2/2018 was performed. Eight patients (1M:7F, average age 62) with HA-PV malformations were identified. Four (50%) patients had a history of liver transplant. All HA-PV malformations were Yakes type IIIA (multiple inflow arteries with a single vein outflow and with the nidus located within the vein wall). In all cases, computed tomography angiography/magnetic resonance angiography was unable to distinguish AVMs from AVFs, and a wrong diagnosis was made in each instance.
Results
Review of pre-procedural Doppler ultrasounds in all cases demonstrated arterialization of portal vein waveforms. Review of pre-procedural cross-sectional (CT/MR) imaging in all of these cases demonstrates a network of arteries around the portal vein with early portal vein filling in every instance. Attempts to close the shunts via arterial inflow embolization but without venous nidus occlusion were performed and were unsuccessful in five out of eight (62.5%) cases. All curative therapies were via embolization of the outflow vein (segmental or lobar portal vein). Technical success was seen in seven of eight cases (87.5%), while one patient is planned to receive additional nidal vein embolization. Liver function was preserved after treatment without worsening of bilirubin or albumin levels.
Conclusion
The diagnosis of an acquired HA-PV malformation can guide curative endovascular treatment by embolization of the portal vein outflow.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arteriovenous malformations (AVMs) have been defined as a vascular structural anomaly involving abnormal arteriovenous (AV) communications bypassing the capillary system [1]. Pathological arterial growth into venous spaces without normal intervening capillary network creates a complex AV shunt. The term malformation implies a congenital etiology and may be defined as a localized morphogenesis error in the first 8 weeks post-conception. However, while AVMs are typically congenital, they may also be acquired [2].
The management of congenital AVMs have been previously described [3,4,5]. Hepatic congenital AVMs are rare with very few cases reported. These patients typically present with portal hypertension (GI bleeding), malabsorption, and failure to thrive in infancy [6]. Most have been treated with hepatic arterial embolization and/or arterial ligation with limited success and recurrences sometimes requiring liver transplantation [7].
There are minimal existing data on hepatic arterial–portal venous (HA-PV) malformations. For the purposes of this manuscript, the term “acquired HA-PV malformation” will be used to represent cases where the following criteria are met: (a) There is no direct single arterioportal communication (no direct arterioportal shunt); (b) there is a complex intervening network of HA-PV communications (nidus) (Fig. 1); and (c) preceding imaging of the area is unremarkable with the absence of vascular abnormality. Using the International Society for the Study of Vascular Anomalies (ISSVA) classification, these lesions would be classified as simple high-flow AVMs. Using the catheter-based Angiographic Classification of AVMs based on Nidus Morphologic Structure, they would be identified as Type II (multiple arterioles shunt into a single venous component) [8]. As per the Yakes classification of AVMs, these would be classified as Yakes Type IIIA with multiple inflow arteries into an aneurysmal vein with single enlarged vein outflow and the nidus present in the vein wall [9].
A Pretreatment ultrasound image of patient 3 demonstrating arterial waveform in the right portal vein. B Angiographic image demonstrating complex arterial network arising from two hypertrophied arterial branches. C Completion angiogram after portal vein embolization [via a left portal vein access (arrow points to coil along the shaft of the hepatic arterial microcatheter which is in the liver parenchymal tract from a prior intervention)] and plug/coil embolization (arrowhead) with decreased AP shunting. D Second procedure with Onyx embolization of two dominant feeder arteries and the previously coiled venous outflow with direct transhepatic puncture
A Pretreatment arterial phase (from triphasic liver CT) image of patient 6 demonstrating right acquired hepatic HA-PV malformation. B Angiographic image demonstrating complex arterial network arising from multiple arterial branches. C Portal vein access and embolization (via the TIPS: arrow) followed by plug embolization of the main trunk of the right portal vein (percutaneous transhepatic access: arrowhead). D Onyx embolization (percutaneous transhepatic access) of the right portal vein with subsequent resolution of AP shunting. E Follow-up angiogram with complete resolution of the AP shunting
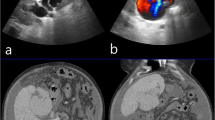
A Pretreatment CTA image of patient 2 demonstrating complex bilobar acquired HA-PV malformation. B Cone-beam CT image demonstrating complex left lobe arterial network (tortous hepatic artery: arrow head) with PV filling (arterialized portal vein: arrow). C 3-Dimensional image demonstrating a complex network of arteries extending to the periphery of the liver with portal vein filling
This study aims to: (a) identify and characterize suspected acquired hepatic arterial–portal venous (HA-PV) malformations and (b) provide guidance for endovascular treatment options.
Materials and Methods
This retrospective review was performed after obtaining institutional review board (IRB) approval. Eight cases of acquired hepatic arterial–portal vein (HA-PV) malformation were identified after reviewing all patients who underwent embolization for hepatic arteriovenous communications (September, 2011 to February, 2018). These cases were reviewed by two independent board-certified interventional radiologists with 3 and 22 years of experience, respectively.
The baseline features of the patients are summarized in Table 1. There were seven females and one male with a median age of 62 years (range 47–69). All patients had imaging features of cirrhosis (liver nodularity) and portal hypertension (splenomegaly, varices). All patients had remote imaging (> 2 years from diagnosis of acquired HA-PV malformations) that demonstrated no HA-PV shunting.
Four of the eight patients had undergone prior liver transplantation. Two of these four presented had elevated bilirubin (one patient had ischemic cholangiopathy) following liver transplantation leading to cross-sectional imaging which demonstrated abnormal HA-PV communications. One patient had AP shunting identified incidentally on routine imaging, and one patient presented with heart failure. Two of the four patients who had prior liver transplantation had documented hepatic arterial anastomotic stenosis which was managed via angioplasty and stent placement.
Two patients had prior TIPS and these patients presented with persistent esophageal varices despite having a TIPS. MR/CT demonstrated abnormal HA-PV communications in each case. One patient had prior partial hepatectomy for metastatic colorectal cancer. This patient presented with varices, and abnormal HA-PV communication was noted on subsequent ultrasound and confirmed by CT. One patient had no prior procedure and presented with varices; abnormal HA-PV communication was noted on CT.
Imaging was reviewed at a multidisciplinary liver conference with hepatologists, transplant surgeons, and interventional radiologists present. All patients had pre-procedural ultrasounds which were reviewed to identify arterialization of portal venous flow which suggested an abnormal HA-PV communication. All patients had contrast-enhanced CT or MRI prior to the angiogram/embolization. Early filling of the portal vein on arterial phase imaging suggested an abnormal HA-PV communication. Angiography was recommended in these cases.
Technique
All eight patients underwent endovascular treatment which involved both superselective hepatic arteriography via a femoral approach and portal venous catheterization and venography either by direct percutaneous transhepatic puncture or via a previously placed TIPS.
On treatment day, hepatic arteriography and portal venography were performed to evaluate the HA-PV malformation. In each instance, dilated portal vein branches in the affected hepatic segment or lobe due to HA-PV shunting were seen. During portal venography, rapid washout of the portal vein by arterialized flow was seen in each case making malformation outflow vein (nidus) determination difficult. For this reason, highly selective arteriography helped identify which portal vein branches were involved, thereby guiding portal vein branch vessel access, catheterization, and embolization.
PV embolization was performed in a segmental fashion when possible. In cases where more extensive embolization was needed, right or left lobar portal vein embolization was performed depending on location of HA-PV malformation.
All direct percutaneous PV access cases were performed under monitored anesthesia care (MAC) or general anesthesia. At the end of the procedure, in cases of direct percutaneous portal vein access, embolization of the hepatic parenchymal tract was performed with coils under sonographic and/or fluoroscopic guidance to prevent bleeding from the liver capsule puncture site.
Outcomes
The procedure was considered a technical success when there was complete absence of HA-PV shunting on post-embolization arteriography. Post-procedural cross-sectional imaging was reviewed, when available, to assess therapeutic success. CT and MRI are limited in assessing therapeutic success as there is artifact from the coils/plugs and glue/onyx. Clinical outcomes were evaluated to assess for resolution of signs/symptoms (improvement in bilirubin and albumin, heart failure, variceal bleeding). Laboratory values were assessed within 3 months following embolization. Complications were assessed following the Cardiovascular and Interventional Radiological Society of Europe classification [10].
Results
Pre-procedure Imaging
HA-PV communications were identified in six out of eight cases by the diagnostic radiologists and misinterpreted as arteriovenous fistulae in every case. None were prospectively identified correctly as HA-PV malformations. Review of pre-procedural Doppler ultrasounds in all cases demonstrated arterialization of portal vein waveforms (Fig. 2). Ultrasound suggested a direct single communication in all cases. Review of pre-procedural cross-sectional (CT/MR) imaging in all of these cases demonstrates a network of arteries around the portal vein with early portal vein filling in every instance (Figs. 3, 4).
Procedure(s)
The procedural details are given in Table 2. Six patients had three procedures which included diagnostic angiographies. One patient had two procedures. One patient had just one procedure. Curative portal vein embolization was preceded by selective (subsegmental/segmental) unsuccessful arterial feeder embolization with coils, glues, and/or Onyx in five out of eight cases.
A combined hepatic arterial and portal venous approach was used with selective or superselective arterial catheterization and arteriography guiding subsequent percutaneous (7/8 patients) and/or TIPS-based (2/8 patients) portal vein access and subsequent PV embolization. The affected PV branches were dilated in all patients.
Portal vein embolization was performed in a segmental fashion in each case. In those malformations where more extensive embolization was needed, right (2/8 patients) or left (3/8 patients) lobar portal vein embolization was performed. Coils/plugs were used in all cases. Onyx was used in two of the eight cases.
Follow-up
Follow-up details are presented in Table 3. None of the patients had a clinically significant worsening of liver function (bilirubin improved or remained stable). Technical success as determined by conventional angiography (elimination of AP shunting) was achieved in seven out of eight cases. One patient had persistent AP shunting from another segment and will be brought back for additional intervention as parastomal variceal bleeding persists. This patient had recurrent bleeding from parastomal varices (with an appropriately functional TIPS), suggesting persistent AP shunting. Seven out of eight patients had follow-up Doppler ultrasound as detailed in Table 3 with hepatopetal PV flow and without evidence of arterialization of PV waveforms.
Clinical outcomes were also evaluated. The patient presenting with heart failure had improvement in cardiac function post-embolization. Two out of four patients with varices had no further variceal bleeding after embolization.
Complications
One patient had a groin pseudoaneurysm which resolved with ultrasound guided compression. No patients had worsening of liver function (hyperbilirubinemia) after embolization. One patient had worsening of preexisting renal dysfunction after the procedure which improved with conservative measures and did not require dialysis.
Discussion
Hepatic arterial–portal venous (HA-PV) fistulae are direct communications between a hepatic artery and a portal vein and most commonly happen due to trauma. Given the increased role of percutaneous therapies in hepatobiliary pathology, iatrogenic HA-PV fistulae are routinely identified. These AVFs are typically managed with coil/plug embolization of the arterial branch which directly communicates with the portal veins [11]. Acquired HA-PV malformations are far more complex than simple HA-PV fistulae and cannot be managed by arterial embolization alone.
The diagnosis of HA-PV malformations on imaging requires awareness of the following findings: (a) arterialization of PV waveform on Doppler imaging; (b) early filling of portal vein on arterial phase of CT or MR imaging; (c) complex hypertrophied arteries around the portal vein (even in the periphery of the liver) and; (d) dilated portal vein branch/branches. The complex hypertrophied vessel (artery) network seen around the portal vein is the cross-sectional imaging finding seen in these lesions which most easily differentiates them from simple AVFs. On angiography, any portal vein filling during selective hepatic arterial angiography is pathologic. (This imaging finding can also be observed in patients with malignant portal vein thrombosis and HA-PV fistulae.) However, acquired HA-PV malformations can be diagnosed if multiple hypertrophied hepatic arterial branches supplying outflow portal vein(s) can be identified. Examples are presented in Figs. 2 and 3.
In our experience, embolization of the arterial inflow has not been successful. This is likely due to the inability to treat the nidus of the HA-PV malformation from the arterial approach. Embolization of one arterial branch can lead to recruitment of other arterial branches.
Definitive therapy (with complete HA-PV shunting resolution) requires nidal occlusion which in this entity means outflow PV catheterization and occlusion. This necessitates portal venous access in every case. Portal vein access can be obtained in the lobe ipsilateral or contralateral to the HA-PV malformation. In patients who have an existing TIPS, the portal vein can be accessed from the transjugular approach. Embolization of the venous outflow (using coils, plugs, or liquid embolic agents such as Onyx and NBCA) is the preferred treatment as recommended by Yakes et al. [9]. Liquid embolic agents may be needed if there is persistent HA-PV shunting despite coil and plug embolization of the outflow PV. Care should be taken to prevent non-target embolization of liquid embolic materials (HA-PV malformation leads to hepatofugal flow in the PV). A combination of plug/coil embolization in the more central aspect of the PV outflow followed by liquid embolic embolization in the peripheral aspect of the lesion prevents non-target embolization.
Portal vein embolization is well tolerated due to the dual nature of hepatic blood supply (arterial and portal). Embolization in a segmental or lobar fashion resulted in preserved portal venous flow to the remaining non-embolized segments. Arterial flow to the region of the liver undergoing portal vein embolization improves preserving viability of these segment(s). Once portal vein embolization has been performed, tract embolization in the hepatic parenchymal tract is needed to decrease risk of post-procedural bleeding (Fig. 2).
The pathophysiology of acquired HA-PV malformations is unknown. We propose two possible processes: (a) neoangiogenesis initiated by an ischemic episode (given two patients had documented hepatic arterial anastomotic stenosis), (b) a post-thrombotic phlebitis process similar to that proposed in the formation of dural AVFs (i.e., venous thrombosis leading to inflammation with subsequent hypertrophy of the vasa vasorum). Interestingly, none of our patients had documented portal venous thrombosis although there may well have been subclinical segmental PV thrombotic episodes which initiated the process: [12, 13]. (c) Additionally, only one patient in this cohort had no history of hepatic intervention (liver transplant/TIPS). The impact of these prior interventions on the development of these acquired hepatic AVMs is unknown.
The peribiliary plexus (PBP) has been described by Kobayashi et al. [14] and represents the small vessels supplying the biliary tree. Cirrhosis causes hypertrophy of the PBP. In addition to the PBP, there is also a periportal plexus (PPP) which represents the vasa vasorum of the portal veins. Ischemia due to decreased arterial flow may lead to hypertrophy of the PPP (to increase portal flow to the liver) eventually leading to the formation of acquired HA-PV malformations (Fig. 5).
The term “acquired AVM” has been described in cases of dural arteriovenous fistulae (AVFs) and uterine AVMs [15]. Dural AVFs are thought to be the result of neoarterial ingrowth into the wall of the dural sinus due to any source of inflammation (thrombosis, trauma). This process may happen in as little as a few days. Meningeal arteries supplying the walls of the dural venous sinuses hypertrophy due to inflammation and arteriovenous shunts are created [15]. In the case of acquired uterine AVMs (post-curettage, C-section, uterine instrumentation, etc.), patients present with menorrhagia/metrorrhagia and historically the treatment was hysterectomy. Many of the lesions have a Yakes Type IIIa/b morphology including an aneurysmal venous outflow. Transcatheter arterial embolization has been effective, but recurrences can occur and some authors have suggested that embolization of the venous outflow nidus is needed for definitive therapy [16].
This retrospective case review describes the diagnosis and management of HA-PV malformations. This study is limited as: (a) it is a retrospective single-institution analysis; (b) changes in PV pressures were not measured before and after embolization; and (c) long-term follow-up data for these patients are also lacking.
Conclusion
This manuscript describes the diagnosis and treatment (embolization of PV outflow) of acquired HA-PV malformations. Future studies should focus on long-term data collected prospectively.
Abbreviations
- AVM:
-
Arteriovenous malformations
- AV:
-
Arteriovenous
- TIPS:
-
Transjugular intrahepatic portosystemic shunt
- PV:
-
Portal vein
- HA-PV:
-
Hepatic arterial–portal vein
- AP:
-
Arterioportal
- MAC:
-
Monitored anesthesia care
- PBP:
-
Prebiliary plexus
- AVF:
-
Arteriovenous fistula
References
Lee BB, Baumgartner I, Berlien HP, Bianchini G, Burrows P, Do YS, et al. Consensus document of the International Union of Angiology (IUA)-2013. Current concept on the management of arterio-venous management. Int Angiol. 2013;32:9–36.
Gnannt R, Clemens RK, Pfammatter T. Transvenous embolization of an acquired arteriovenous malformation of the arm. J Vasc Interv Radiol. 2015;26:1585–7. https://doi.org/10.1016/j.jvir.2015.03.024.
Cho SK, Do YS, Kim DI, Kim YW, Shin SW, Park KB, et al. Peripheral arteriovenous malformations with a dominant outflow vein: results of ethanol embolization. Korean J Radiol. 2008;9:258–67. https://doi.org/10.3348/kjr.2008.9.3.258.
Cho SK, Do YS, Shin SW, Kim DI, Kim YW, Park KB, et al. Arteriovenous malformations of the body and extremities: analysis of therapeutic outcomes and approaches according to a modified angiographic classification. J Endovasc Ther. 2006;13:527–38. https://doi.org/10.1583/05-1769.1.
Hwang JH, Do YS, Park KB, Chung HH, Park HS, Hyun D. Embolization of congenital renal arteriovenous malformations using ethanol and coil depending on angiographic types. J Vasc Interv Radiol. 2017;28:64–70. https://doi.org/10.1016/j.jvir.2016.09.004.
Sutcliffe R, Mieli-Vergani G, Dhawan A, Corbally M, Karani J, Heaton N. A novel treatment of congenital hepatoportal arteriovenous fistula. J Pediatr Surg. 2008;43:571–3. https://doi.org/10.1016/j.jpedsurg.2005.07.005.
Fellows KE, Hoffer FA, Markowitz RI, O'Neill JA Jr. Multiple collaterals to hepatic infantile hemangioendotheliomas and arteriovenous malformations: effect on embolization. Radiology. 1991;181:813–8. https://doi.org/10.1148/radiology.181.3.1947103.
Dunham GM, Ingraham CR, Maki JH, Vaidya SS. Finding the Nidus: detection and workup of non-central nervous system arteriovenous malformations. Radiographics. 2016;36:891–903. https://doi.org/10.1148/rg.2016150177.
Yakes WF, Yakes AM. Classification of arteriovenous malformation and therapeutic implication. In: Mattassi R, Loose D, Vaghi M, editors. Hemangiomas and vascular malformations. Milano: Springer; 2015, pp. 263–276. https://doi.org/10.1007/978-88-470-5673-2_33.
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the Cirse classification system. Cardiovasc Intervent Radiol. 2017;40:1141–6. https://doi.org/10.1007/s00270-017-1703-4.
Choi BI, Lee KH, Han JK, Lee JM. Hepatic arterioportal shunts: dynamic CT and MR features. Korean J Radiol. 2002;3:1–15. https://doi.org/10.3348/kjr.2002.3.1.1.
Kerber CW, Newton TH. The macro and microvasculature of the dura mater. Neuroradiology. 1973;6:175–9.
Link DP, Garza AS, Monsky W. Acquired peripheral arteriovenous malformations in patients with venous thrombosis: report of two cases. J Vasc Interv Radiol. 2010;21:387–91. https://doi.org/10.1016/j.jvir.2009.10.035.
Kobayashi S, Nakanuma Y, Matsui O. Intrahepatic peribiliary vascular plexus in various hepatobiliary diseases: a histological survey. Hum Pathol. 1994;25:940–6.
Wanke I, Rufenacht DA. The Dural AV-Fistula (DAVF), the most frequent acquired vascular malformation of the central nervous system (CNS). Clin Neuroradiol. 2015;25(Suppl 2):325–32. https://doi.org/10.1007/s00062-015-0449-0.
Picel AC, Koo SJ, Roberts AC. Transcatheter arterial embolization with n-butyl cyanoacrylate for the treatment of acquired uterine vascular malformations. Cardiovasc Intervent Radiol. 2016;39:1170–6. https://doi.org/10.1007/s00270-016-1328-z.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Riaz, A., Vogelzang, R., Young, V. et al. Endovascular Management of Acquired Hepatic Arterial–Portal Venous Malformations. Cardiovasc Intervent Radiol 43, 466–477 (2020). https://doi.org/10.1007/s00270-019-02380-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02380-w