Abstract
Background and Aims
Hepatic encephalopathy (HE) is a common complication of elective transjugular intrahepatic portosystemic shunt (TIPS) placement and is often successfully medically managed. Risk factors for refractory hepatic encephalopathy (RHE) necessitating revision of TIPS are not well defined. We evaluated the incidence, predictors, and outcomes of post-TIPS RHE necessitating TIPS revision.
Methods
In a retrospective cohort study of 174 consecutive patients undergoing elective TIPS placement (2010–2015), we evaluated the incidence of post-TIPS RHE. Clinical demographics and procedural variables were collected. 1-year outcomes after revision were collected.
Results
Ten of 174 patients (5.7%) developed post-TIPS RHE requiring revision. Significant differences between RHE and non-refractory groups were shunt size > 8 versus ≤ 8 mm (18.5 vs. 3.4%, p = 0.001), history of HE (14 vs. 2%, p = 0.007), and serum albumin levels ≤ 2.5 versus > 2.5 g/dL (13.1 vs. 3.1%, p = 0.020). On multivariate analysis, shunt size > 8 mm (p = 0.001), history of HE prior to TIPS (p = 0.006), and low serum albumin (≤ 2.5 g/dL) (p = 0.022) remained independent predictors of RHE, controlling for age and Model for End-Stage Liver Disease score. RHE improved in 8 of 10 patients but survival at 1 year without liver transplantation (LT) was only 10%.
Conclusion
While TIPS revision successfully improves RHE in most cases, 1-year mortality rates are high, limiting the value of revision in non-LT candidates. Patients with previous history of HE and low serum albumin levels prior to TIPS may benefit most from the use of shunt sizes < 8 mm to mitigate the risk of RHE.
Level of Evidence
Level 4, case series.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transjugular intrahepatic portosystemic shunt (TIPS) placement is an established treatment option for complications of cirrhosis related to portal hypertension [1]. With technical refinements, peri-procedural complications and mortality from TIPS have decreased [2]. Long-term complications including need for rescue transplant, shunt dysfunction/need for revision, and mortality have also decreased with the advent of polytetrafluoroethylene (PFTE) covered stents [3, 4]. However, hepatic encephalopathy (HE) remains one of the most common and significant complications of TIPS with an estimated incidence of 18–45% depending on the exact definition of HE used and patient demographics [5,6,7]. The postulated mechanism is multi-factorial including increased production of enteric neurotoxins from intestinal bacteria, reduced hepatic filtering due to liver dysfunction or vascular shunting from the portal to systemic circulations and increased permeability of the blood–brain barrier [8, 9]. One large meta-analysis found age over 65, advanced Child–Pugh score, and history of HE to be robust predictors of post-TIPS HE [10].
The incidence and risk factors for post-TIPS refractory hepatic encephalopathy (RHE) are less well characterized. The reported incidence has ranged from 3 to 8% although little is known about demographic risk factors specific to this complication [11,12,13,14,15,16]. The aim of this investigation was to determine the incidence and risk factors for post-TIPS RHE after elective TIPS placement. We also evaluated outcomes after TIPS shunt revision as a treatment for post-TIPS RHE.
Materials and Methods
Subject Inclusion/Exclusion
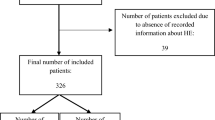
After institutional review board approval, consecutive patients who underwent TIPS from January 2010 to December 2015 at a tertiary referral hospital were identified by procedural (ICD-9/ICD 10) code. Retrospective medical record review was performed identifying 273 individuals who underwent initial TIPS placement during the timeframe. Seventy-nine patients had non-elective TIPS for endoscopically uncontrolled variceal hemorrhage, and 20 patients with incomplete data were excluded leaving 174 patients for final analysis.
Clinical Aims
The primary aim of investigation was incidence of post-TIPS RHE. This was operationally defined as new or worsening HE within 1 year of TIPS that either: (1) required hospital admission with no improvement in clinical status despite at least 72 h of medical therapy (combination of lactulose per os/per rectum and rifaximin 550 mg twice daily per os) and exclusion of precipitating events: infection, gastrointestinal bleeding, azotemia, acute or acute-on-chronic portal vein thrombus, polypharmacy or (2) required 3 or more hospitalizations over a subsequent 3-month period for post-TIPS HE [17,18,19]. Subjects’ outpatient and inpatient records including initial admission assessments and discharge diagnoses were reviewed. Clinical demographics including age, gender, race, pre-procedure Model for End-Stage Liver Disease (MELD) score, hemoglobin, creatinine, international normalized ratio (INR), total bilirubin, albumin, history of pre-procedure HE, and TIPS indication were collected and tabulated.
Initial TIPS Placement and Subsequent Revision for Refractory Hepatic Encephalopathy
Procedural variables from the initial TIPS placement including hepatic venous wedge pressure, direct portal venous pressures, direct right atrial pressures, portosystemic pressure gradient (PSG), and shunt size were recorded. Direct portal and direct right atrial pressures were obtained post-stent placement and final dilation at the termination of the procedure. Procedural data from the revision procedure were recorded as well: PSG before and after revision and shunt size. Outcome parameters assessed included presence/absence of improvement in HE, 90-day readmission rate for HE, liver transplant (LT) rates, and death up to 1 year after TIPS revision. Relevant clinical data at the time of revision were gathered to allow for calculation of total number of organ failures (OF) and CLIF-ACLF (Chronic Liver Failure Consortium Acute-on-Chronic Liver Failure) scores for each patient [20].
Procedure Descriptions
Initial TIPS Placement
TIPS was performed utilizing Ring Transjugular Intrahepatic Access Set with Colapinto needle (Cook Medical, Bloomington, IN, USA), typically from the right hepatic vein to the proximal right portal vein. Prior to 2012 procedures were performed with fluoroscopic guidance with wedged carbon dioxide (CO2) portovenograms, parallax, and occasionally assisted by transabdominal ultrasound and/or direct portal vein micropuncture access/wire placement to facilitate access [21, 22]. Post-2012 majority of procedures were performed with intravascular ultrasound guidance [23]. Transluminal angioplasty of the intrahepatic tract followed by deployment of a Viatorr (Gore Medical, Flagstaff, AZ, USA) covered stent across the tract was carried out. All stents had a nominal diameter of 10 mm. Appropriate length of stents was determined by simultaneous portovenogram (utilizing a marker band flush catheter) and inferior venocavogram via the 10-French angle-tipped ring set sheath. Stents were ideally placed from the portal vein extending slightly into the inferior vena cava. Most stents were dilated initially to 8 mm, and pressures were measured. If PSG was greater than 12 mmHg, then the stents were further dilated to 9 or 10 mm to achieve a PSG of less than 12 mmHg. A small number of patients with preexisting HE were only dilated initially to 6 mm. Repeat PSG, right atrial pressure, and a venogram were obtained after balloon dilation of the stent.
Stent Reduction A parallel reduction technique was used [15]. Venous access was gained through the right jugular vein. A catheter was advanced into the TIPS stent for venography, portal venous pressure, and right atrial pressure measurements. A guidewire was left in place. A second access site in the right jugular vein was then gained to allow for passage of a second guidewire across the TIPS stent. A balloon expandable express SD stent (Boston Scientific, Boston, MA, USA) was placed over one guide wire, and a Viatorr covered stent was placed over the other guidewire. With 2 parallel stents in place, they were simultaneously expanded in the middle of the original TIPS stent, forming a waist. The degree of dilation of the Express SD stent was adjusted to reach an end PSG of 12 mmHg and achievement of antegrade portal flow on venogram. Repeat portal venous, right atrial pressure, and a venogram were obtained after balloon dilation.
Stent Closure Access to the TIPS stent was gained in a similar fashion. Venogram and pressure measurements were taken. An Amplatzer plug (St Jude Medical, St Paul, MN, USA) was then deployed in the existing stent [15]. Repeat venogram confirmed complete occlusion.
Statistical Analysis
Variables including age, gender, race, pre-procedure MELD score, hemoglobin, creatinine, INR, total bilirubin, albumin, history of pre-procedure HE, TIPS indication, and presence of portal vein thrombus (PVT) were analyzed to determine predictors of readmission for post-TIPS RHE. We used logistic regression to determine independent predictors of RHE. Initially, we performed univariate logistic regression to determine significant predictors of RHE followed by multivariate logistic regression controlling for other variables in the model and age. The significance was determined by a p value of less than 0.05 in both univariate and multivariate analysis. For other demographic variables, we conducted t test and Chi-square test to determine mean and distribution difference, respectively. All analyses were performed using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA).
Results
Patient Demographics
Patient demographics and baseline laboratory values for the 174 patients undergoing elective TIPS are shown in Table 1. Men outnumbered women approximately 2:1. Mean age was 56.6 ± 10.3 years, and average MELD score was 13.5 ± 4.4. Baseline laboratory data were notable for an average creatinine of 1.0 ± 0.3 mg/dL, albumin of 3.0 ± 0.6 g/dL, total bilirubin of 2.0 ± 1.7 mg/dL and INR of 1.5 ± 0.3. Fifty patients (29%) had a history of HE prior to TIPS controlled with lactulose. Indications for TIPS were refractory ascites in 79 patients (45%), hepato-hydrothorax 25 patients (14%), non-bleeding varices in 26 patients (15%), and other in 44 patients (25%). Seventy-one patients (41%) had non-occlusive PVT without extension into the superior mesenteric or splenic vein prior to TIPS.
Procedural Variables
At time of initial TIPS, mean initial PSG was 16.4 ± 5.0 mmHg, final PSG was 7.2 ± 2.5 mmHg, and PSG difference was 9.3 ± 4.0 mmHg. Forty-five patients (26%) had a final PSG of ≤ 5 mmHg.
Refractory Hepatic Encephalopathy and Risk Factors
Ten of 174 patients (5.7%) developed RHE requiring TIPS reduction (n = 9) or closure (n = 1). Median time to revision procedure was 55 days (range 15–409). By study inclusion criteria, all patients had at least 1 OF (RHE). Seven patients met criteria for ACLF with 2 or more OF using CLIF-C ACLF scores at the time of revision (Table 3). Five had 2 OF; the remaining 2 patients had 3 and 4 OF. Average CLIF-C ACLF score of the 7 patients was 50.1 (range 44–57). Average initial PSG was 8.6 mmHg (range 4–16). After the 9 reduction procedures, final PSG increased to a mean of 13 mmHg (range 7–20).
Demographic and procedural variables for the groups with and without RHE are presented in Table 2. On univariate analysis, shunt size > 8 versus ≤ 8 mm (18.5 vs. 3.4%, p = 0.001), history of HE prior to TIPS (14 vs. 2%, p = 0.007), and serum albumin levels ≤ 2.5 vs. > 2.5 g/dL (13.1 vs. 3.1%, p = 0.020) were associated with higher incidence of RHE. Low serum albumin level as a continuous variable was found to be a significant predictor of RHE as well (p = 0.005). However, age, gender, MELD score, indication for procedure, and presence of PVT did not affect the risk of RHE. Furthermore, no procedural hemodynamic variable was predictive of RHE. Of note, no patient who developed RHE had a final gradient of ≤ 5 mmHg. On multivariate analysis, shunt size > 8 mm (p = 0.001), history of HE prior to TIPS (p = 0.006), and serum albumin ≤ 2.5 g/dL (p = 0.022) remained independent predictors of RHE, controlling for age and MELD score.
Outcomes of TIPS Reduction/Closure
All patients were continued on lactulose and rifaximin post-revision. Improvement in RHE was seen in 8 of 10 patients (80%). Only one patient after initial improvement developed subsequent grade 2 HE requiring re-hospitalization within 90 days. The patient was admitted a month after undergoing TIPS reduction from a skilled nursing facility where she developed constipation from inappropriate lactulose dosing. She improved rapidly with titration of lactulose and had no further admissions for HE.
Outcome data are presented in Table 3. LT-free survival was only 10% at 1 year (1 of 10 patients). Four patients (40%) underwent successful LT at a median of 12.5 days (range 3–126) after TIPS revision. Five patients (50%) were not deemed candidates for LT and died within 1 year of TIPS revision. The 2 patients with 3–4 OF had no significant improvement after TIPS revision; 1 died and 1 required LT within 15 days. There was no good correlation in outcome between patients with 1 versus 2 OF.
Discussion
Post-TIPS HE is a common and significant complication after TIPS placement. In most cases, it can be managed successfully with treatment of any precipitating events and medical therapy with lactulose and rifaximin [24]. However, less commonly HE persists or is recurrent despite maximal medical therapy and requires more complex management.
In our study, 10 of 174 patients (5.7%) developed RHE after initial elective TIPS placement. This was consistent with prior studies who noted an incidence of post-TIPS RHE of 3–8% [11,12,13,14,15,16]. In the largest single-center series addressing RHE, Kochar et al. [11] found 38 of 733 patients (5.2%) undergoing TIPS over a 14-year period developed RHE requiring TIPS revision. They noted a potential association between older age and RHE but a formal analysis of risk factors for RHE was not the focus of their study. In a retrospective study of HE after TIPS with PTFE grafts, Riggio et al. [25] noted 6 of 78 patients (8%) developed HE. Increased creatinine level was the only variable independently associated with RHE. Other studies to date have not found any predictors for the development of RHE.
Unique to our study is the finding that shunt size > 8 mm, history of HE prior to TIPS, and low serum albumin (≤ 2.5 g/dL) were significant predictors of RHE. Each of these variables has been previously shown to be risk factors for HE but not in the RHE population. A recent trial showed a higher incidence of post-TIPS HE in patients randomized to receive a 10 versus 8 mm covered stent during TIPS for variceal prevention [26]. History of HE prior to TIPS is one of the most well-known, robust predictors of HE. Low albumin has been shown in 2 prior to studies to be a predictor of HE [27]. A recent study by Nardelli found that sarcopenia was an independent risk factor for post-TIPS HE [28]. Likewise, decreased muscle mass was shown to worsen rates of minimal HE and overt HE in a prospective study of cirrhotic patients not undergoing TIPS [29]. This link is possibly due to the involvement of skeletal muscle in ammonia metabolism [30]. As low serum albumin (≤ 2.5 g/dL) was predictive of RHE while MELD score was not in our study, this difference could have been driven by malnutrition. Numerous studies have also demonstrated that malnutrition and sarcopenia are risk factors for multiple other complications and death [27].
Of note, no hemodynamic variables were found to predict RHE, including final PSG. A prior study noted a final PSG of ≤ 5 mmHg to predict low-pressure gradient-related complications, defined as death, RHE or deterioration of hepatic function [13]. In our study, the average final PSG was 8.1 mmHg and no patient with a final PSG ≤ 5 mmHg developed RHE.
Nine patients underwent TIPS shunt reduction and 1 patient underwent closure. RHE initially improved in 8 of 10 patients (80%). However, long-term outcomes were poor. Four patients underwent transplant, 3 of whom occurred within 2 weeks of shunt reduction. Of the remaining 6 patients, 90-day and 1-year survival was only 50 and 10%, respectively. Kochar et al. noted poorer outcomes in patients who developed RHE within 90 days of TIPS and in patients who did not respond to shunt revision. More complications related to increased portal hypertension were seen in patients undergoing shunt closure (n = 29) versus shunt reduction (n = 9) [11]. A more recent article by Schultheiss et al. also noted relatively poor outcomes after TIPS revision/closure in 17 patients. Mortality at 4 weeks was 30 and 47% at 1 year. Patients who developed RHE in the setting of liver insufficiency have a particularly poor prognosis [16]. Five of our 10 patients (50%) had early RHE which could account for our lower transplant-free survival rates. The 2 patients with CLIF-C ACLF scores of 3 and 4 had no significant benefit from the procedure. Proceeding to immediate transplant or addressing goals of care in non-LT candidates could avoid subjecting these patients with severe ACLF to futile procedures. In stark comparison, overall 1-year transplant-free survival after TIPS was found to be 88% in one study [31]. Therefore, preventing post-TIPS RHE appears critical.
There was variability in the final PSG at time of initial TIPS placement or balloon dilation of TIPS stenosis and the initial PSG at time of TIPS revision. Overall PSG decreased by a mean of 0.5 mmHg with 3 patients developing gradients of 4 mmHg (2 with 8 mm shunts, 1 with a 10 mm shunt). Part of this difference could be contributed to the recognized phenomenon of passive expansion of the stent beyond the size of balloon dilation at the time of stent placement [32]. Of the 5 patients with initial shunt dilation to 8 mm, the average decrease was slightly higher at 1.2 mmHg. However, it is unlikely this small difference negates our findings on multivariate analysis.
Our study does have limitations. Data were collected retrospectively and from chart review. Inclusion was based on admit and discharge diagnoses in patient records which could have been inaccurate or incomplete as we also were only able to capture patients admitted post-procedure at our medical center. It is also not possible to ensure the development of RHE was a complication of TIPS and not natural progression of liver disease. However, to avoid this, we chose a 1-year follow-up period and 5 of 10 patients developed RHE within 90 days of initial TIPS. We also were unable to assess the incidence of grade 0 or 1 HE before or after TIPS and TIPS revision as we do not routinely monitor for this. Furthermore, the degree of stent balloon dilation accounted for factors such as history of HE. Given a balloon dilation to 6 mm was initially used in these patients, we likely underestimate the harm of larger diameter shunts. It is likely that more frequent use of stents < 8 mm would have been beneficial, especially in high-risk patients (low albumin, prior HE, older age, high bilirubin), as 5 of 10 patients who developed RHE had 8 mm stents. Finally, the operational definition of RHE was arbitrarily chosen to be 72 h from admission—this was based on our center’s anecdotal clinical experience in management admissions for hepatic encephalopathy. Treatment with lactulose and rifaximin on uniform dosing and schedule was used in all patients which limits treatment bias.
Conclusion
In conclusion, RHE is a relatively uncommon but severe complication after TIPS. While TIPS revision successfully improves RHE in most cases, long-term outcomes are very poor without LT. Limiting shunt size, especially in patients with a history of HE or low serum albumin, may mitigate the risk of post-TIPS RHE.
References
Parker R. Role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Clin Liver Dis. 2014;18:319–34.
Perry BC, Kwan SW. Portosystemic shunts: stable utilization and improved outcomes, two decades after the transjugular intrahepatic portosystemic shunt. J Am Coll Radiol. 2015;12:1427–33.
Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157–63.
Garcia-Pagan JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–9.
Casadaban LC, Parvinian A, Minocha J, et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015;60:1059–66.
Riggio O, Nardelli S, Moscucci F, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Clin Liver Dis. 2012;16:133–46.
Sauerbruch T, Mengel M, Dollinger M, et al. Prevention of rebleeding from esophageal varices in patients with cirrhosis receiving small-diameter stents versus hemodynamically controlled medical therapy. Gastroenterology. 2015;149:660–8.
Garcovich M, Zocco MA, Roccarina D, et al. Prevention and treatment of hepatic encephalopathy: focusing on gut microbiota. World J Gastroenterol. 2012;18:6693–700.
Garcia-Pagan JC, Di Pascoli M, Caca K, et al. Use of early TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol. 2013;58:45–50.
Bai M, Qi X, Yang Z, et al. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: a systemic review. J Gastroenterol Hepatol. 2011;26:943–51.
Kochar N, Tripathi D, Ireland H, Redhead DN, Hayes PC. Transjugular intrahepatic portosystemic stent shunt (TIPPSS) modification in the management of post-TIPSS refractory hepatic encephalopathy. Gut. 2006;55:1617–23.
Fanelli F, Salvatori FM, Rabuffi P, et al. Management of refractory hepatic encephalopathy after insertion of TIPS: long-term results of shunt reduction with hourglass-shaped balloon-expandable stent-graft. AJR Am J Roentgenol. 2009;193:1696–702.
Chung HH, Razavi MK, Sze DY, et al. Portosystemic pressure gradient during transjugular intrahepatic portosystemic shunt with Viatorr stent graft: what is the critical low threshold to avoid medically uncontrolled low pressure gradient related complications? J Gastroenterol Hepatol. 2008;23:95–101.
Otal P, Smayra T, Bureau C, et al. Preliminary results of a new expanded-polytetrafluoroethylene-covered stent-graft for transjugular intrahepatic portosystemic shunt procedures. AJR Am J Roentgenol. 2002;178:141–7.
Pereira K, Carrion AF, Salsamendi J, et al. Endovascular management of refractory hepatic encephalopathy complication of transjugular intrahepatic portosystemic shunt (TIPS): comprehensive review and clinical practice algorithm. Cardiovasc Intervent Radiol. 2016;39:170–82.
Schultheiss M, Bettinger D, Boettler T, et al. Severe hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: value of shunt reduction and occlusion. JSM Hepatol. 2017;2:1009–15.
Cordoba J, Ventura-Cots M, Simon-Talero M, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure. J Hepatol. 2014;60:275–81.
Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American association for the study of liver diseases and the European association for the study of liver disease. Hepatology. 2014;60:715–35.
Villa E, Camma C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and hepatic decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253–60.
Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;60:275–81.
Loffroy R, Estivalet L, Cherblanc V, et al. Transjugular intrahepatic portosystemic shunt for the management of acute variceal hemorrhage. World J Gastroenterol. 2013;19:6131–43.
Caporossi JM, Vidal V, Jacquier A, et al. Balloon occlusion versus wedged hepatic venography using iodinated contrast for targeting the portal vein during TIPS. Diag Interv Imaging. 2015;96:357–63.
Kao SD, Morshedi MM, Narsinh KH, et al. Intravascular ultrasound in the creation of transhepatic portosystemic shunts reduces needle passes, radiation dose, and procedure time: a retrospective study of a single-institution experience. J Vasc Interv Radiol. 2016;27:1148–53.
Sharma BC, Sharma P, Lunia MK, et al. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458–63.
Riggio O, Nicolao F, Angeloni S, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Incidence and risk factors. Dig Dis Sci. 1996;41:578–84.
Wang Q, Lv Y, Bai M, et al. Eight millimetre covered TIPS does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal bleeding. J Hepatol. 2017;67:508–16.
Anand AC. Nutrition and muscle in cirrhosis. J Clin Exp Hepatol. 2017;7:340–57.
Nardelli S, Lattanzi B, Torrisi S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol. 2017;15:934–6.
Merli M, Gusto M, Lucidi C, et al. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281–4.
Wright G, Noiret L, Olde Dammink SW, Jalan R. Interorgan ammonia metabolism in liver failure: the basis of current and future therapies. Liver Int. 2011;31:163–75.
Bureau C, Garcia-Pagan JC, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicenter study. Liver Int. 2007;27:742–7.
Mollaiyan A, Bettinger D, Rossele M. The underdilation of nitinol stents at TIPS implantation: solution or illusion? Eur J Radiol. 2017;89:123–8.
Funding
This study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Rowley, M.W., Choi, M., Chen, S. et al. Refractory Hepatic Encephalopathy After Elective Transjugular Intrahepatic Portosystemic Shunt: Risk Factors and Outcomes with Revision. Cardiovasc Intervent Radiol 41, 1765–1772 (2018). https://doi.org/10.1007/s00270-018-1992-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-1992-2