Abstract
Purpose
The purpose of this study was to assess the technical feasibility of ultrasound-guided endovascular creation of a percutaneous extraluminal arterial bypass graft without a surgically created arterial anastomosis.
Materials and Methods
Nine swine were utilized for this IACUC-approved study using a carotid bypass model in swine. Using sonographic guidance, percutaneous access was obtained to the proximal and distal common carotid artery. A self-expanding stent graft was advanced through the proximal carotid access site, tunneled subcutaneously, then advanced through the distal carotid access site, and deployed. The stent grafts were monitored weekly for patency using ultrasound. Angiography was performed at 4 weeks to evaluate for graft patency. Gross pathologic analysis was performed on the explanted stent grafts.
Results
In eight out of the nine swine (89%), percutaneous extraluminal bypass graft creation was technically successful, with brisk flow through the stent graft to the distal circulation, complete exclusion of the bypassed segment of carotid artery, and no extravasation. The technical failure was due to stent graft maldeployment. Of the six swine evaluated for patency, four grafts were patent at the 4-week end point. Both occluded stent grafts were due to extraluminal extrusion of one end, likely related to neck movement and growth in neck length.
Conclusion
The percutaneous arterial bypass technique had a high technical success rate and a graft patency rate of 67% at 4 weeks, with early occlusions possibly related to limitations of the animal model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The standard treatment of severe occlusive peripheral arterial disease (PAD) of the superficial femoral artery (SFA) is surgical extra-anatomic vein bypass grafting [1, 2]. However, the PAD patient population frequently has multiple medical comorbidities such as chronic obstruction pulmonary disease (COPD), diabetes mellitus, hypertension, and coronary artery disease, which may render them poor candidates for general anesthesia and surgery. Furthermore, some patients are not candidates for bypass due to anatomic or technical limitations, such as a hostile groin or excessive arterial calcifications [3].
Percutaneous endovascular techniques for treatment of stenosis and occlusions of the SFA have demonstrated a high short-term success rate and a long-term patency similar to prosthetic bypass grafts [4,5,6,7,8] and have the advantage of a shorter recovery time, less pain, and lower risks associated with moderate sedation when compared to general anesthesia. However, not all arterial occlusions can be recanalized with endovascular techniques. In addition, severely diseased arteries with bulky calcifications may not respond well to stent deployment, due to incomplete stent expansion [9]. Without an effective revascularization treatment for patients who are neither surgical nor endovascular candidates, amputation may be required. The purpose of this study was to assess the technical feasibility of endovascular creation of a percutaneous extraluminal arterial bypass graft without a surgically created arterial anastomosis.
Materials and Methods
Animal Model
The experimental procedure was performed in a swine model following protocol approval by the institutional animal care and use committee. A total of nine female domestic Yorkshire swine initially weighing 36–42 kg were utilized for this study. The animals were fed a standard swine diet and were housed in the institutional animal facility. After arrival, they were allowed to acclimate to the facility for at least 3 days before the procedure was performed. One practice swine was used for optimization of technique and was sacrificed immediately following the procedure.
Preprocedural Preparation
Aspirin (325 mg PO) was administered daily starting at least 3 days prior to the procedure and continuing throughout the postoperative course until animal kill. A fentanyl patch (50 mcg/h) was placed on the swine the night before the procedure for pain control. Peri-procedural antibiotic prophylaxis was utilized, consisting of a single dose of intravenous cefazolin (25 mg/kg). The veterinary anesthesia team induced general anesthesia in each swine. Ketamine 22 mg/kg IM and acepromazine 1.1 mg/kg IM were used for initial sedation. Isoflurane 0–4.5% inhaled continuously after intubation was used for general anesthesia.
Percutaneous Arterial Bypass Graft Technique
All procedures were performed by an attending interventional radiologist with 6 years of experience. Grayscale and color Doppler ultrasound were used to identify the length of the left common carotid artery. Using sonography, appropriate proximal and distal access sites with a relatively superficial location of the left common carotid artery and minimal intervening musculature were identified. Using sterile technique, local anesthesia was induced using a subcutaneous injection of bupivacaine (0.25%). A 5-mm skin nick was created at both access sites. Under sonographic guidance, the proximal left common carotid artery was accessed retrograde at an approximately 45° angle using a 21-gauge needle (Micropuncture set; Cook, Bloomington, IN), allowing insertion of the 5-French micropuncture sheath oriented toward the heart. An angiogram was performed through the micropuncture sheath under fluoroscopy to delineate the anatomy of the left common carotid artery. The distal left common carotid artery was then accessed with a micropuncture kit in the anterograde direction under sonographic guidance with the micropuncture catheter pointing toward the head. The proximal and distal left common carotid artery micropuncture access sheaths were each exchanged for a 7-French peel-away sheath over their respective guidewires, oriented toward the heart proximally and away from the heart peripherally. A heparin bolus was then administered intravenously (100–200 units/kg).
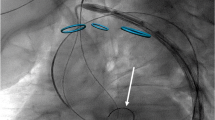
The right common femoral artery was then accessed with a micropuncture set, allowing insertion of a 7-French vascular sheath into the right common femoral artery over a guidewire. A 10-mm goose-neck snare was advanced into the abdominal aorta. A 0.035″ exchange length guidewire was inserted through the proximal left common carotid sheath into the mid-abdominal aorta. The guidewire was snared and pulled out through the right common femoral artery sheath to obtain through-and-through access between this and the proximal left common carotid sheath. The subcutaneous soft tissues between the proximal and distal skin nicks at the left neck were then gently dissected with a curved hemostat to prepare for the creation of the subcutaneous bypass graft. A 5 mm × 10 cm or 6 mm × 10 cm stent graft (GORE® VIABAHN® Endoprosthesis, Gore, Flagstaff, AZ) was then inserted over the guidewire through the right common femoral artery vascular sheath and advanced over the guidewire until it abutted the dilator within the proximal left carotid peel-away sheath. The central introducer was then backed out as the stent graft was advanced through the peel-away sheath to avoid significant hemorrhage. The end of the stent graft was passed through the subcutaneous tunnel. The dilator within the distal carotid peel-away sheath was removed, and the tip of the stent graft was immediately inserted into the peel-away sheath. Under fluoroscopic observation, the guidewire was then advanced into the distal common carotid artery to the branch point. The stent graft was then advanced approximately 1–2 cm into the distal common carotid artery. The position of the stent graft was evaluated using fluoroscopy to ensure that the arteriotomy sites were covered. Both peel-away sheaths were removed while applying pressure to the arterial access sites and the stent graft was immediately deployed. Angioplasty was performed throughout the stent graft with a 5 or 6 mm diameter x 4 cm length angioplasty balloon (Powerflex, Cordis, Hialeah, FL) in order to seal the edges of the stent graft, dilate both arteriotomies, and disrupt any septa within the subcutaneous tunnel. Follow-up carotid angiography was performed to assess stent graft patency, to confirm complete occlusion of the excluded segment of common carotid artery, and to evaluate for evidence of extravasation at the arteriotomy sites (Fig. 1). The catheters and sheaths were removed, and hemostasis of the right common femoral artery was achieved with manual compression. The skin nicks at the neck were closed with a single buried absorbable suture.
Percutaneous creation of an extraluminal subcutaneous arterial bypass graft in swine using a carotid artery bypass model in animal #9. A A digitally subtracted image demonstrates the proximal and distal carotid artery access sites. The micropuncture sheath directed cranially is annotated with the arrowhead; the micropuncture sheath directed towards the heart is annotated with an arrow, B after deployment of the stent graft, left carotid arteriography demonstrates patency of the stent graft, lack of extravasation, complete exclusion of the bypassed segment, and preserved distal perfusion. Note arterial vasospasm in distal carotid artery (arrow). The focal angulation in the stent graft (arrowhead) is related to the fluoroscopic obliquity in the craniocaudal dimension. C Repeat left carotid arteriogram at 4 weeks demonstrates patency of the bypass graft with maintained distal perfusion. Two mild intra-stent stenoses are present in the proximal stent graft (arrows)
Post-procedural Follow-Up
Following the procedure, the swine continued to receive 325 mg of aspirin daily by mouth. The arterial bypass grafts were monitored for patency using weekly Doppler ultrasound. Angiography was performed at 4 weeks to assess graft patency and to evaluate for evidence of stenosis or other complications. Peri-stent stenoses were defined as stenoses limited to within 1 cm from the stent graft margin. Intra-stent stenoses were defined as stenoses within the stent graft, greater than 1 cm from the stent graft margin. If the bypass graft was occluded on Doppler ultrasound evaluation, the swine underwent evaluation by angiography followed by euthanasia earlier than 4 weeks. After follow-up angiography, the animals were euthanized by injection of potassium chloride while sedated. Dissection was carried down to the stent graft site to evaluate the positioning of the stent graft and the presence of any hematoma. The stent grafts were then surgically excised and visually inspected for evidence of intraluminal thrombus or fibrous intimal hyperplasia.
Results
Technical Success
The percutaneous creation of a subcutaneous extraluminal arterial bypass graft was technically successful in eight of the nine swine (89%) (Table 1). All successfully bypassed animals demonstrated brisk flow through the stent graft, complete exclusion of the bypassed segment of carotid artery, no extravasation at the arteriotomy sites, and preservation of arterial flow to the distal circulation. Mild vasospasm was noted immediately adjacent to the stent graft in all animals. The one technical failure (swine 4) was due to inadvertent extraluminal positioning of one stent graft margin, which resulted in active extravasation and stent graft thrombosis. This animal was immediately euthanized.
Complications
The swine that underwent stent graft maldeployment was euthanized immediately. One swine demonstrated seizure-like activity approximately 45 min after the procedure then expired soon after, potentially due to embolic stroke, anesthesia complications, or unrelated pathology. Swine number nine was febrile on post-procedure day 20, and ultrasound of the neck demonstrated a small complex fluid collection near the stent graft in the soft tissues of the left neck, which was compatible with a hematoma, which was potentially infected. The swine was treated empirically with antibiotics, and the fever resolved. The fluid collection was no longer visualized on ultrasound a week later.
Patency Evaluation
Of the 9 initial swine utilized, one was a planned non-survival animal intended for technical refinement. Two were excluded due to peri-procedural complications as described above. Of the remaining six swine evaluated for short-term patency, serial weekly Doppler ultrasound examinations demonstrated that five swine had patent bypass grafts at one week. Angiography of the swine without Doppler flow signal (swine 6) confirmed that the bypass graft was occluded with extraluminal extrusion of the inferior margin of the stent graft, which was confirmed on gross pathologic evaluation. At weeks 2 and 3, the remaining five swine demonstrated patent bypass grafts. Angiography at the 4-week endpoint demonstrated that four swine had patent bypass grafts. One swine (swine 3) was found to have bypass graft occlusion on angiography, again with apparent extraluminal extrusion of the inferior aspect of the stent graft, which was confirmed on gross pathologic inspection. Over the 4-week study period, the average swine weight increased from 41.0 to 64.4 kg.
Of the 4 swine with patent bypass grafts undergoing angiography at 4 weeks, all bypass grafts demonstrated in-stent stenosis ranging from 10 to 90% (Fig. 2). Two had solely intra-stent stenosis, one had solely a peri-stent stenosis, and one had both intra- and peri-stent stenosis (Table 1). On gross pathologic examination, the three stent grafts with intra-stent stenosis visualized on angiography all had gross evidence of smooth fibrous intimal hyperplasia that corresponded with the degree of stenosis seen on angiography. No intraluminal or wall-adherent thrombus was identified within any of the excised stent grafts (Figs. 2 and 3).
Evaluation of stent graft-associated stenoses in animal #8 at the 4-week endpoint. A A severe intra-stent stenosis is present at the proximal aspect of the stent graft (arrow) with a moderate peri-stent stenosis distally (arrowhead). Note the minimal overlap of the stent graft with the artery at the level of the arrow, which we feel may be responsible for the two instances of stent graft extrusion from the artery. B Gross pathologic examination demonstrates dense white fibrous intimal hyperplasia without adherent thrombus, which corresponds to the proximal stenosis (arrow)
Discussion
The current study demonstrated the technical feasibility of entirely percutaneous creation of a subcutaneous extraluminal arterial bypass graft, with technical success achieved in eight out of nine animals. This study also demonstrated that the percutaneously created stent graft bypass can maintain short-term patency, with two-thirds of stent grafts patent at 4 weeks. The two stent grafts that were occluded on follow-up angiography appear to have extruded one margin extraluminally secondary to either neck movement or substantial growth in neck length over the weeks following the procedure (the average swine weight increased by 57% over the 4-week study duration in these juvenile animals). The investigated technique could theoretically provide an alternative treatment option for patients failing conventional therapies who are otherwise resigned to amputation. Endovascular therapies are infeasible for lesions that cannot be recanalized. Additionally, lesions that re-occlude soon after intervention may be poor candidates for repeat intervention. Surgical bypass graft creation can be difficult or impossible in patients with extensive scar tissue, extensive mural calcifications, or persistent infectious complications involving the common femoral artery. Considering that over 800,000 lower extremity amputations were performed in the USA related to peripheral arterial disease in 2005, development of additional strategies to prevent or delay amputation is important [10].
To date, the entirely percutaneous creation of an extraluminal arterial bypass conduit has not been reported in the literature in living animals or humans, although related techniques have been reported. Percutaneous creation of a transrenal arteriovenous hemodialysis graft was previously reported in a swine model, which demonstrated the feasibility of percutaneous creation of an arterial “anastomosis” using a stent graft, without evidence of extravasation on angiography [11]. The deployment of an in situ balloon-expandable endoluminal endograft has been trialed in humans, but with poor mid- and long-term patency rates [12]. Other novel approaches to re-route arterial flow have been described, such as percutaneous creation of arteriovenous fistulae and endovascular venous arterialization [13, 14]. In humans, the endovascular creation of an extraluminal femoropopliteal bypass graft was proven to be technically feasible in an ex vivo human cadaver model [15]. A recently developed percutaneous bypass system (PQ Bypass Inc, Sunnyvale, CA) utilizes the adjacent femoral vein as conduit within which to deploy stent grafts that bypasses an arterial occlusion. Notably, sacrifice of the femoral vein may have clinical consequences, particularly if the greater saphenous vein was already harvested as conduit material. Additionally, patients with venous pathology would not be ideal candidates for this approach.
Although this technique is conceptualized for use in the setting of SFA occlusions in humans, the carotid artery provides a more favorable setting for investigating arterial bypass grafts in swine for several reasons and is therefore ubiquitous within the surgery literature [16,17,18,19]. Firstly, animals tend to scratch and chew accessible parts of their body when irritation is present. A bypass graft in the animal’s leg could be prone to damage by chewing on the overlying skin, which could affect the integrity and patency of the stent graft. Secondly, the musculature about the proximal lower extremity artery in swine is extensive, which would require the bypass graft to traverse a thick muscular layer. In humans, surgical arterial bypass grafts are implanted without traversing major muscle bellies. Thirdly, the caliber of the human SFA is approximately 5–6 mm, which is similar to that of the swine carotid artery.
All 4 swine with patent bypass grafts undergoing final 4-week angiography demonstrated in-stent stenosis. In two animals, the in-stent stenosis was mild and occurred near the stent graft margins, which is typical for intimal hyperplasia migrating from the vessel lumen. Three animals demonstrated in-stent stenosis remote from the stent graft margins, which has been reported to be secondary to focal thrombus formation on the stent graft wall with smooth muscle adherence and subsequent organization and matrix development [20, 21]. Both of these phenomena are intrinsic limitations of stent grafts [22]; however, in humans, in-stent stenosis rarely occurs to such an extreme degree so quickly; thus, this extremely rapid and severe stenosis formation may not be necessarily generalizable to humans, considering that swine arteries develop stent-associated thrombus, inflammation, and intimal hyperplasia at an accelerated rate compared to humans [23]. The rapid rate and severity of intimal hyperplasia formation encountered in this study are similar to that reported in other animal studies of arterial stent grafts in swine models. For example, a prior study on GORE® VIABAHN® Endoprosthesis implantation in the carotid artery of swine demonstrated a mean residual luminal diameter of 67% after only two weeks secondary to intimal hyperplasia [24], which is markedly accelerated compared to what occurs in humans [25]. These findings also suggest that additional pharmacologic strategies, such as clopidogrel therapy, may be helpful for optimization of the efficacy of this technique. Further evolution of this technique may also include development of specialized equipment for this purpose. While longer stent grafts may be well suited for this technique, traditional bypass graft conduit bonded to self-expanding stent grafts at both ends could serve this solution well. In the USA, there is already a commercially available arterial bypass graft that has one margin bonded to a stent graft (GORE® Hybrid Vascular Graft). Tunneling of the stent graft or conduit could be easily performed using conventional subcutaneous tunneling methods. As demonstrated by this study, the bypassed arterial segment is completely excluded from the arterial circulation, which would be inconsequential for bypassing an arterial occlusion; however, if the arterial entry site is adjacent to an artery branch, such as the deep femoral artery or the anterior tibial artery, deployment of the stent graft could result in occlusion of those artery origins.
As a feasibility study, this study is limited by the small sample size, which prevents generation of robust conclusions and subanalyses. While the swine carotid bypass model is commonly utilized in the surgery literature to evaluate new techniques and materials planned for eventual use in the human lower extremity, this model in growing swine demonstrated limitations for assessing the proposed endovascular technique. The potential for growth in neck length was not taken into account, which may have contributed to stent graft extrusion from the lumen. In future animal studies using the carotid artery model, a longer stent graft with more extension into the carotid artery may mitigate this phenomenon. Additionally, the neck is a site of substantial rotational mobility; we hypothesize that repetitive twisting of the neck over time may have encouraged migration of one end of the stent graft out of the lumen of the artery in this swine carotid bypass model. A stent graft overlap margin of 2 cm or more may prevent this phenomenon. In the human adult, an increase in leg length would not be an issue, and while there is minimal rotational motion involving the SFA in humans, the popliteal artery may be prone to the effect of stent graft dislodgement. This study is also limited by the short-term follow-up for patency. However, swine models are limited for long-term patency evaluation of stents and bypass grafts due to the markedly accelerated intimal hyperplasia that is highly dissimilar to human physiology. A minor limitation is the lack of formal histologic analysis, as only gross examination of the explanted stent graft was performed. However, histologic features of arterial stent grafts in animal models have been well characterized [24], with the classic gross findings of a glistening white layer lining the stent graft corresponding to intimal hyperplasia.
In summary, this study demonstrated the technical feasibility of a percutaneous extraluminal arterial bypass graft in an in vivo swine model without using a surgical arterial anastomosis. These results warrant further investigation of the early- and long-term patency rates in a more suitable animal model, as it holds promise as a potential option for patients with severe PAD who have failed or are poor candidates for endovascular interventions and conventional surgical bypass.
References
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–67.
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654.
Bouman E, Dortangs E, Buhre W, Gramke HF. Current techniques and strategies for anesthesia in patients undergoing peripheral bypass surgery. J Cardiovasc Surg (Torino). 2014;55:207–16.
Ichihashi S, Higashiura W, Itoh H, Sakaguchi S, Nishimine K, Kichikawa K. Long-term outcomes for systematic primary stent placement in complex iliac artery occlusive disease classified according to Trans-Atlantic Inter-Society Consensus (TASC)-II. J Vasc Surg. 2011;53:992–9.
Park KB, Do YS, Kim DI, Kim DK, Kim YW, Shin SW, et al. The TransAtlantic InterSociety Consensus (TASC) classification system in iliac arterial stent placement: long-term patency and clinical limitations. J Vasc Interv Radiol. 2007;18:193–201.
Koizumi A, Kumakura H, Kanai H, Araki Y, Kasama S, Sumino H, et al. Ten-year patency and factors causing restenosis after endovascular treatment of iliac artery lesions. Circ J. 2009;73:860–6.
Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–34.
McQuade K, Gable D, Pearl G, Theune B, Black S. Four-year randomized prospective comparison of percutaneous ePTFE/nitinol self-expanding stent graft versus prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. J Vasc Surg. 2010;52:584–90 (discussion 590–581, 591.e581–591.e587).
Chang IS, Park KB, Do YS, Park HS, Shin SW, Cho SK, et al. Heavily calcified occlusive lesions of the iliac artery: long-term patency and CT findings after stent placement. J Vasc Interv Radiol. 2011;22:1131–7.
Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–9.
Wallace MJ, Middlebrook M, Wright KC. Creation of a transrenal arteriovenous dialysis shunt: feasibility study in a swine model. J Vasc Interv Radiol. 2001;12:1325–32.
Heijmen RH, Teijink JA, van den Berg JC, Overtoom TT, Pasterkamp G, Moll FL. Use of a balloon-expandable, radially reinforced ePTFE endograft after remote SFA endarterectomy: a single-center experience. J Endovasc Ther. 2001;8:408–16.
Rajan DK, Ebner A, Desai SB, Rios JM, Cohn WE. Percutaneous creation of an arteriovenous fistula for hemodialysis access. J Vasc Interv Radiol. 2015;26:484–90.
Schreve MA, Unlu C, Kum S, Tan YK. Surgical and endovascular venous arterialization: ready to take the “desert” by storm? J Cardiovasc Surg (Torino). 2017;58:402–8.
Maynar M, Llorens R, Uson-Gargallo J, Crisostomo V, Lopez-Sanchez C, Garcia-Martinez V, et al. Endovascular placement of an extraluminal femoropopliteal bypass graft in human cadavers. Cardiovasc Interv Radiol. 2005;28:209–14.
Kappert U, Ouda A, Virmani R, Mettler D, Matschke K, Demertzis S. The C-Port xV(R) vascular anastomosis system: results from an animal trial. Thorac Cardiovasc Surg. 2011;59:222–6.
Kapadia MR, Aalami OO, Najjar SF, Jiang Q, Murar J, Lyle B, et al. A reproducible porcine ePTFE arterial bypass model for neointimal hyperplasia. J Surg Res. 2008;148:230–7.
Abi-Jaoudeh N, Pritchard WF, Amalou H, Linguraru M, Chiesa OA, Adams JD, et al. Pulsed high-intensity-focused US and tissue plasminogen activator (TPA) versus TPA alone for thrombolysis of occluded bypass graft in swine. J Vasc Interv Radiol. 2012;23:953–61.
Begovac PC, Thomson RC, Fisher JL, Hughson A, Gallhagen A. Improvements in GORE-TEX vascular graft performance by Carmeda BioActive surface heparin immobilization. Eur J Vasc Endovasc Surg. 2003;25:432–7.
Cejna M, Virmani R, Jones R, Bergmeister H, Loewe C, Schoder M, et al. Biocompatibility and performance of the Wallstent and the Wallgraft, Jostent, and Hemobahn stent-grafts in a sheep model. J Vasc Interv Radiol. 2002;13:823–30.
Gordon BM, Fishbein MC, Levi DS. Polytetrafluoroethylene-covered stents in the venous and arterial system: angiographic and pathologic findings in a swine model. Cardiovasc Pathol. 2008;17:206–11.
Geraghty PJ, Mewissen MW, Jaff MR, Ansel GM, Investigators V. Three-year results of the VIBRANT trial of VIABAHN endoprosthesis versus bare nitinol stent implantation for complex superficial femoral artery occlusive disease. J Vasc Surg. 2013;58:386–95.
Touchard AG, Schwartz RS. Preclinical restenosis models: challenges and successes. Toxicol Pathol. 2006;34:11–8.
Wong G, Li JM, Hendricks G, Eslami MH, Rohrer MJ, Cutler BS. Inhibition of experimental neointimal hyperplasia by recombinant human thrombomodulin coated ePTFE stent grafts. J Vasc Surg. 2008;47:608–15.
Saxon RR, Chervu A, Jones PA, Bajwa TK, Gable DR, Soukas PA, et al. Heparin-bonded, expanded polytetrafluoroethylene-lined stent graft in the treatment of femoropopliteal artery disease: 1-year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) trial. J Vasc Interv Radiol. 2013;24:165–73 (quiz 174).
Acknowledgements
This work was funded by the RSNA Research Resident Grant #RR1564 to Jessica Stewart.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Stewart, J.K., Perkins, S.S. & Kim, C.Y. Creation of an Extraluminal Arterial Bypass Graft Using a Commercially Available Self-Expanding Stent Graft: Feasibility Study in a Porcine Model. Cardiovasc Intervent Radiol 40, 1447–1453 (2017). https://doi.org/10.1007/s00270-017-1672-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1672-7