Abstract
Aim
To evaluate the technical success and mid-term outcomes following transcatheter embolisation of type 1a endoleak after Nellix endovascular aneurysm sealing (EVAS).
Materials and Methods
Seven patients (5 men; mean age 83; range 79–90) underwent transcatheter embolisation between July 2013 and August 2014. The average time from EVAS to embolisation was 136 days (range 6–301) and from endoleak diagnosis to embolisation was 20 days (range 2–50). Embolisation was performed with coils and Onyx in six cases and Onyx only in one case. Technical success, imaging and clinical outcomes of embolisation were reviewed. Technical success was defined as elimination of the endoleak on completion angiography and first imaging follow-up. Clinical success was defined as unchanged or decreased aneurysm sac size on subsequent follow-up (average 8 months; range 103–471 days).
Results
All cases were technically successful. One patient required a second endovascular procedure following Onyx reflux into the Nellix endograft and another patient required surgical closure of a brachial puncture site. All patients are endoleak free with stable sac size on the latest available follow-up imaging.
Conclusion
If a type 1 endoleak occurs after EVAS, embolisation using Onyx with or without coils is feasible and effective with high technical success and freedom from endoleak recurrence at mid-term follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular abdominal aortic aneurysm repair (EVAR) has almost superseded open surgery for anatomically suitable patients [1]. The Nellix endovascular aneurysm sealing (EVAS) system (Endologix, Santa Rosa, CA) is a novel endograft, which in contrast to conventional EVAR devices comprises two balloon expandable stents, each surrounded by a polymer filled endobag, which conform to the shape of the patients aneurysm thereby providing aneurysm exclusion.
Type 1a endoleak (EL1a) is one of the leading factors necessitating secondary intervention following conventional EVAR [2]. The incidence, significance and optimal management of EL1a following Nellix EVAS is not known. We describe the first reported case series of EL1a embolisation following Nellix EVAS using Ethylene vinyl alcohol copolymer (Onyx—Covidien, Irvine, California, USA) alone or with coils, and discuss considerations in the technical approach.
Materials and Methods
This is a single center retrospective observational study conducted at a tertiary referral center. From July 2013 to August 2014, 7 patients (5 men, 2 women; mean age 83; range 79–90 years) underwent embolisation for EL1a following Nellix EVAS. Prior to EVAS, three patients had proximal neck anatomy that were outside the manufacturer’s guidelines for instructions for use (IFU) for Nellix EVAS due to short or very angulated necks, two patients had borderline neck anatomy according to the IFU for the same reasons, and in two patients the endografts were deployed slightly below the renal arteries, which is thought to have been the cause of the endoleak. An endoleak was not identified on completion angiography following EVAS in any of the cases.
All CT scans were reviewed by a senior vascular interventional radiologist with more than 20 years experience. Aneurysm sac diameter was measured at the largest anterior-posterior axial dimension. Duplex US scans were performed by specialised vascular sonographers. All cases were reviewed in a multi-disciplinary meeting prior to proceeding with embolisation. Indication for transarterial embolisation was the presence of a EL1a on CTA or Duplex US.
Technical success was defined as elimination of the endoleak on completion angiography and first imaging follow-up. Clinical success was defined as absence of increased aneurysm sac size (>5 mm) or recurrence of endoleak on subsequent follow-up. Follow-up imaging was performed by Duplex US in all cases and additional CTA in four of the seven cases. Two patients did not undergo post-embolisation CTA due to renal function impairment.
CTA is performed using the GE VCT 64 slice scanner (GE Healthcare, Waukesha, WI, USA) following administration of 90 ml non-ionic contrast medium 300 mgI/ml at 5 ml/s via a power injector into an 18G venous access catheter in the antecubital vein. Scans are extended from the thoracic inlet to the common femoral artery (CFA) bifurcation and scan delay is determined by bolus tracking over a region of interest over the aorta.
Transarterial Embolisation Procedure
Prior to arterial access, the CTA is evaluated to assess the position of the endograft and the site of access most favourable for accessing the endoleak. For example, an endoleak on the right of the aortic sac is best accessed from a left CFA approach, since the endografts cross over at the bifurcation and thus the left iliac limb graft is located on the right in the upper abdominal aorta.
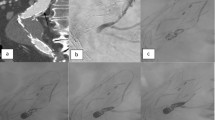
Common femoral or occasionally brachial artery access is obtained. A 45 cm straight 6F sheath is placed with the sheath tip a few centimeters below the top of the endograft. AP (anteroposterior) and lateral aortograms are performed using a flush catheter paced above the proximal end of the endografts to assess the size, geometry and neck of the endoleak. An on-table rotational angiographic CT (Dyna-CT, Siemens, Erhlangen, Germany) may be useful for additional information regarding the size and anatomical configuration of the endoleak and how to access it. The entrance into the endoleak is catheterized using a reverse shaped [e.g. 5F Sim 1 (Cook Inc., Bloomington, IN, USA)] catheter in all cases except when a brachial access is used (patient 7), where a hockey stick shaped [e.g. 5F Dav (Cook Inc., Bloomington, IN, USA)] catheter is utilised. After engaging the endoleak with the catheter, an ‘endoleakogram’ is performed. A microcatheter (2.7F Progreat (Terumo Corporation, Somerset, New Jersey) or 2.95F PX SLIM (Penumbra, Alameda, California) is advanced co-axially under fluoroscopic visualisation into the endoleak cavity. In six of seven of cases, embolisation was performed with detachable coils (e.g. Ruby, Penumbra Inc., Almeda, CA) before the injection of Onyx. The authors prefer to use only detachable retrievable coils because of the potential for coil misplacement and migration out of the endoleak cavity. Once the endoleak space is sufficiently filled with coils so that the coils form a scaffold defining the outline of the endoleak cavity, Onyx 34 is injected into the interstices between the coils to provide complete occlusion of the endoleak cavity. Onyx is injected slowly under intermittent frequent fluoroscopic monitoring, as there is a significant risk of Onyx reflux; particularly in wide-necked endoleaks (e.g. >10 mm). Injection of Onyx is stopped upon complete filling of the endoleak cavity. A completion aortogram is performed to assess for residual endoleak filling. Figure 1 illustrates the steps involved in this technique. After the procedure, patients were discharged from hospital the following day and were followed up clinically and by interval Doppler ultrasound and CTA.
Technique for embolisation of EL1a following Nellix EVAS. A Coronal CTA image shows finger-like projection of contrast between the left Nellix endograft and the aortic wall. B Aortic angiogram in anterio-posterior projection via flush catheter placed above the endograft shows the EL1a along the lateral aspect of the left endograft. Note the Nellix endografts cross over at the bifurcation and therefore the left iliac limb endograft is seen on the right in the abdominal aorta. In this case, left CFA access was obtained to access the endograft on the right of the abdominal aorta; which would provide the easiest access to the endoleak on the basis of the CTA. C Endoleakogram performed after engaging the endoleak entrance with a reverse shaped catheter better shows the size and morphology of the endoleak. D The endoleak cavity is filled with detachable coils to form a scaffolding. E Onyx injected into the interstices between the coils to provide complete occlusion of the endoleak cavity. F Completion angiography shows no residual endoleak
Results
Procedural Outcome
Proximal type 1 endoleaks were confirmed and embolised in seven patients in a total of seven procedures. The average aneurysm sac size prior to embolisation was 7 cm (range 5.9–9.4). Endoleak diagnosis was based on CTA in six patients and on Duplex in one patient. The average time between EVAS and endoleak diagnosis was 104 days (range 1–421). The average time from Endoleak diagnosis to embolisation was 20 days (range 2–50) (Table 1). In six cases, CFA access was used, and left brachial access was used in one patient. Detachable microcoils (Two to five 0.18 coils ranging between 4 and 12 mm), and Onyx (2–10 ml depending on the size of the endoleak) were used for embolisation in six cases and Onyx alone in one case. Immediate technical success with complete isolation of the endoleak was achieved in all cases.
There were three procedural complications, none with long-term sequelae. In the case where brachial access was used (patient 7), haemostasis could not be achieved despite prolonged manual compression and the patient required surgical closure of the arterial puncture. In the first embolisation case performed (patient 1), Onyx without coils was used and towards the end of the embolisation, reflux of some Onyx into the left Nellix endograft was observed. Completion angiography showed patency of the endografts with satisfactory elimination of the endoleak. However, a subsequent CTA 6 weeks post-embolisation showed >75 % stenosis of the left Nellix limb by the Onyx. This was successfully alleviated by placement of a covered stent within the Nellix limb; affixing the Onyx between the stent and the inside wall of the Nellix endograft; and almost fully restoring stent patency. This case has recently been published as a case report [3]. A smaller reflux of Onyx into the right Nellix limb was observed at completion of embolisation in another case (patient 4). In this case the proximal end of the right endograft where Onyx was observed was stented open with a self-expanding stent at the time.
Follow-up
Average imaging follow-up available post-embolisation is 231 days (range 103–471) (Table 2). All endoleaks were occluded at the first imaging follow-up examination. There has been no case of endoleak recurrence or increase in sac size on post-embolisation imaging. Therefore, both the technical and clinical success rates for embolisation of EL1a after EVAS are 100 %.
The two cases (patients 1 and 4) with Onyx reflux during embolisation show complete stent patency at the latest imaging follow-up with no adverse findings following secondary stent placement (follow-up of 103 and 471 days, respectively). The patient who developed haematoma requiring surgical closure from a brachial access puncture had almost complete resolution of arm swelling when seen in clinic 2 months post-embolisation.
Discussion
Herein, we have reported the technique and outcomes of embolisation of EL1a in seven patients who had undergone Nellix EVAS.
The incidence and significance of EL1a following Nellix EVAS is unknown with only a handful of cases reported [3–5]. Proximal perigraft endoleak is not uncommon following conventional EVAR occurring in up to 10 % of cases [6], and usually mandates early secondary intervention as the blood flow outside of the endograft lumen is associated with increased sac pressurisation (endotension) and a risk of rupture [7]. However, unlike other devices, the Nellix endograft is based on a sac-anchoring aneurysm sealing mechanism and does not rely on a proximal and distal fixation for sac isolation. The device uses polymer filled endobags, which conform to the shape of the aneurysm and obliterate the aneurysm lumen to achieve a seal thus providing aneurysm exclusion and sealing of side branch flow [8].
If an EL1a occurs after EVAS, blood may flow into the potential space between the endobag and aortic wall. There are no data on the natural history of untreated EL1a after EVAS. However, it is intuitive to predict that EL1a after EVAS has a potential for sac enlargement and ultimately rupture, although neither of these have been reported. For this reason, a policy of early embolisation of these EL1a has been adopted at our institution. Amongst our cohort, in one case (patient 4), where a subtle endoleak was undiagnosed on CTA at 2 and 5 months post-EVAS, there was a significant increase in the endoleak size observed at 10 months (Fig. 2). However, this was not associated with an increase in sac size. In another patient (patient 7), there was a 5 mm increase in sac size observed between the 6-month post-EVAS CTA showing no endoleak and the 9-month scan demonstrating an EL1a. The other 5 patients were diagnosed and embolised very early, and did not undergo sufficient follow-up to assess temporal changes in terms of endoleak and sac size.
Axial A–D and coronal E CTA images at the level of the proximal end of the Nellix endograft at 1 day (A), 2 months (B), 5 months (C) and 10 months (D and E) post-EVAS. A At 1 day, there are satisfactory appearances with no endoleak. Note the normal finding of high density appearance of the endobags (arrow) following Nellix EVAS which can conceal an early endoleak. The observed high density is due to the polymer being mixed with contrast during deployment and diminished with time as the endobags become gradually isodense to the thrombosed aneurysm sac. B and C At 2 and 5 months, respectively, there is a subtle area of high density between the two endobags (arrow head); which was not identified as an endoleak at the time. At 10 months D and E, there is an obvious EL1a which has increased in size significantly compared to the two earlier CT scans. The aortic sac size was unchanged
Standard ‘bail-out’ strategies for EL1a with conventional aortic endografts such as aortic cuffs, and large calibre balloon expandable stents are not feasible after EVAS. Embolisation appears to be the only feasible non-surgical approach for treating such EL1a. The literature on the management of EL1a following conventional EVAR is very limited. Our published experience of embolisation of six type 1 endoleaks, four of which were proximal endoleaks showed 100 % technical success with Onyx embolisation and no recurrent endoleaks at up to 10 months follow-up[9]. There are few other short reports on type 1 endoleak embolisation with Onyx, together comprising 15 patients [6, 10–12].
Although this author routinely uses Onyx alone for the embolisation of type 2 endoleaks and EL1a after EVAR using non-Nellix devices, the EL1a cavity soon after diagnosis in EVAS patients is small and typically has the morphology of a finger-like projection passing inferiorly between the endobag and aortic wall. Therefore, the potential for migration of Onyx out of the endoleak cavity into the aortic lumen during the embolisation procedure may be higher than for EL1a embolisation with non-Nellix aortic endografts. For this reason, the author prefers to initially use detachable coils to form a scaffold in the EL1a cavity before completing the procedure with Onyx.
In conclusion, EL1a after EVAS is very uncommon with only a handful of cases reported. Although the natural history of untreated EL1a after EVAS is not known, embolisation is an effective management choice with a high technical and clinical success.
References
Albuquerque FC, Tonnessen BH, Noll RE, et al. Paradigm shifts in the treatment of abdominal aortic aneurysm: Trends in 721 patients between 1996 and 2008. J Vasc Surg. 2010;51:1348–53.
Conrad MF, Adams AB, Guest JM, et al. Secondary intervention after endovascular abdominal aortic aneurysm repair. Ann Surg. 2009;250:383–9.
Ameli-Renani S, Das R, Weller A, et al. Embolisation of a proximal type I endoleak post-nellix aortic aneurysm repair complicated by reflux of onyx into the nellix endograft limb. Cardiovasc Intervent Radiol 2015;38(3):747–751.
Krievins DK, Holden A, Savlovskis J, et al. EVAR using the Nellix Sac-anchoring endoprosthesis: treatment of favourable and adverse anatomy. Eur J Vasc Endovasc Surg. 2011;42:38–46.
Donayre CE, Zarins CK, Krievins DK, et al. Initial clinical experience with a sac-anchoring endoprosthesis for aortic aneurysm repair. J Vasc Surg. 2011;53:574–82.
Grisafi JL, Boiteau G, Detschelt E, et al. Endoluminal treatment of type IA endoleak with Onyx. J Vasc Surg. 2010;52:1346–9.
Green N, Sidloff DA, Stather PW, et al. Endoleak after endovascular aneurysm repair: current status. Rev Vasc Med. 2014;2:43–7. doi:10.1016/j.rvm.2013.11.002.
Böckler D, Peters AS, Pfeiffer S, et al. Nellix® endovascular aneurysm sealing (EVAS)—a new technology for endovascular management of infrarenal aortic aneurysms. Zentralbl Chir. 2014;139:562–8. doi:10.1055/s-0034-1383084.
Chun J-Y, Morgan R. Transcatheter embolisation of type 1 endoleaks after endovascular aortic aneurysm repair with Onyx: when no other treatment option is feasible. Eur J Vasc Endovasc Surg. 2013;45:141–4.
Eberhardt KM, Sadeghi-Azandaryani M, Worlicek S, et al. Treatment of type I endoleaks using transcatheter embolization with onyx. J Endovasc Ther. 2014;21:162–71.
Henrikson O, Roos H, Falkenberg M. Ethylene vinyl alcohol copolymer (Onyx) to seal type 1 endoleak. A new technique. Vascular. 2011;19:77–81.
Loffroy R, Steinmetz E. Regarding “endoluminal treatment of type IA endoleak with Onyx”. J Vasc Surg. 2011;53:1163 (author reply 1163–4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
‘R.A. Morgan’ is a Proctor for Covidien.
Rights and permissions
About this article
Cite this article
Ameli-Renani, S., Morgan, R.A. Transcatheter Embolisation of Proximal Type 1 Endoleaks Following Endovascular Aneurysm Sealing (EVAS) Using the Nellix Device: Technique and Outcomes. Cardiovasc Intervent Radiol 38, 1137–1142 (2015). https://doi.org/10.1007/s00270-015-1171-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-015-1171-7