Abstract
The objective of this study was to assess prospectively the role of multislice CT angiography (MSCTA) on planning of radiological catheter placement for hepatic arterial infusion chemotherapy (HAIC). Forty-six patients with malignant liver tumors planned for HAIC were included. In each patient, both MSCTA and intra-arterial digital subtraction angiography (DSA) were performed, except one patient who did not undergo DSA. Comparison of MSCTA and DSA images was performed for the remaining 45 patients. Detectability of anatomical variants of the hepatic artery, course of the celiac trunk, visualization scores of arterial branches and interobserver agreement, presence of arterial stenosis, and technical outcome were evaluated. Anatomical variations of the hepatic artery were detected in 19 of 45 patients (42%) on both modalities. The course of the celiac trunk was different in 12 patients. The visualization scores of celiac arterial branches on MSCTA/DSA were 3.0 ± 0/2.9 ± 0.2 in the celiac trunk, 3.0 ± 0/2.9 ± 0.3 in the common hepatic artery, 2.9 ± 0.2/2.9 ± 0.3 in the proper hepatic artery, 2.9 ± 0.3/2.9 ± 0.4 in the right hepatic artery, 2.8 ± 0.4/2.9 ± 0.4 in the left hepatic artery, 2.9 ± 0.2/2.9 ± 0.3 in the gastroduodenal artery, 2.1 ± 0.8/2.2 ± 0.9 in the right gastric artery, and 2.7 ± 0.8/2.6 ± 0.8 in the left gastric artery. No statistically significant differences exist between the two modalities. Interobserver agreement for MSCTA was equivalent to that for DSA. Two patients showed stenosis of the celiac trunk on both modalities. Based on these imaging findings, technical success was accomplished in all patients. In conclusion, MSCTA is accurate in assessing arterial anatomy and abnormalities. MSCTA can provide adequate information for planning of radiological catheter placement for HAIC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatic arterial infusion chemotherapy (HAIC) is an established treatment for unresectable malignant hepatic neoplasm [1–3]. Although a few randomized control trials have shown a survival benefit of HAIC [4, 5], HAIC still remains an important treatment option because of the higher response rate and lower systemic toxicities compared with systemic chemotherapy [1–5]. For the implantation of a catheter and port system, a percutaneous approach using techniques of interventional radiology is now widely used instead of surgical methods [1, 3]. Radiological catheter placement consists of serial procedures including unification of multiple hepatic arteries, embolization of gastrointestinal branches arising from the hepatic artery, and implantation of a catheter and port system. All these procedures are needed to accomplish the adequate distribution of anticancer agents to the entire liver without extrahepatic perfusion through the implanted catheter and port system [1, 3, 6]. Incorrect evaluation of vascular anatomy sometimes leads to inadequate situations such as a defect of drug distribution in the liver, gastrointestinal complication, or catheter-related arterial occlusion resulting in treatment failure. Thus, vascular mapping is essential for planning of radiological catheter placement. Although conventional angiography has been used for such assessment, multislice CT angiography (MSCTA) may be suitable for noninvasive abdominal vascular evaluation [7–10]. However, the specific need for radiological catheter and port placement by interventional radiologists, such as variations of the hepatic artery, gastrointestinal branches arising from hepatic arteries, and the course of the celiac trunk, has not been well evaluated. The purpose of this study was to assess prospectively the role of MSCTA in planning of radiological catheter placement for HAIC.
Materials and Methods
Patients
Between August 2003 and March 2007, eligible patients were enrolled in this study. Eligibility criteria were as follows: (a) patients planned to undergo radiological catheter and port placement for HAIC, (b) patients without prior catheter placement for HAIC or any kind of embolotherapy to the celiac or hepatic arterial region, (c) patients without a history of adverse reaction to contrast material and renal dysfunction (serum creatinine ≥ 2.0), and (d) written informed consent obtained. This study protocol was approved by the institutional review board of our institution.
MSCT Image Acquisition and Postprocessing
A 16-channel multislice CT scanner (Aquilion-16; Toshiba Medical Systems, Tokyo) was used for the evaluation. MSCTA was performed as part of a pretreatment routine CT evaluation of the abdomen. Ninety to one hundred milliliters of nonionic contrast material (iohexol; Omnipaque 300 syringe; Daiichi-Sankyo, Inc., Tokyo) was administered into the antecubital vein at a rate of 3 mL/s with an automated injector (Dual Shot/ A-300; Nemoto Kyorindo, Tokyo). For MSCTA, arterial-phase image scanning was started within 8 s after reaching the bolus of contrast material into the descending aorta (100 HU), using a bolus-tracking method. Images were obtained during inspiration. A collimation of 0.5 mm with a helical pitch of 15 was used. Three-dimensional (3-D) reconstruction was obtained on a dedicated workstation (Zio M900; Amin, Tokyo). For 3-D image reconstruction, multiplanar volume reformation (MPVR) images were obtained. When small vessels, including the right gastric artery, were not visualized on conventional MPVR images, additional images using targeted MPVR technique were used.
Digital Subtraction Angiography
Intra-arterial digital subtraction angiography (DSA) using Seldinger’s technique was performed just before implantation of the catheter and port system, within 2 weeks after MSCTA. A transfemoral 5-Fr simple curved catheter was inserted selectively into the celiac trunk. Twenty to twenty-five milliliters of nonionic contrast material (iopamidol; Iopamiron 300; Bayer-Schering Pharma, Tokyo) was administered at a rate of 4–5 mL/s. Images were obtained during expiration.
Image Interpretation and Evaluation
Four radiologists evaluated MSCTA and DSA images independently, blinded to the other observers’ results or patient history. A 4-week interval was set between the sessions for evaluation of two modalities to eliminate the awareness of the findings of another modality. Arterial anatomy and abnormalities were assessed regarding the following items.
-
1.
Anatomic variation of the hepatic artery
-
2.
Course of the celiac trunk: cranial, horizontal, or caudal
-
3.
Visualization of the following vessels: celiac trunk, common hepatic artery (CHA), proper hepatic artery (PHA), right hepatic artery (RHA), left hepatic artery (LHA), gastroduodenal artery (GDA), right gastric artery (RGA), and left gastric artery (LGA). Visualization scores were on a scale of 1–3 (1, not visualized; 2, fair visualization [i.e., only origin or proximal portion of the artery recognizable]; 3, excellent visualization (i.e., entire course of the artery recognizable). Interobserver agreement was also evaluated.
-
4.
Stenosis of ≥50% in any segment of the hepatic arteries
Estimation of Radiation Dose
Radiation exposure was estimated by measuring the volume computed tomography dose index (CTDIvol) and dose length product (DLP). CTDIw (mGy) was automatically calculated and displayed on the CT scanner using particular parameters for the specific imaging protocol mentioned above. DLP (mGy · cm) was calculated by the following formula [11]:
where scan length (cm) is the length of the scan for individual patients.
Technical Outcome
Technical success was evaluated regarding unification of multiple hepatic arteries, embolization of gastric arteries, and implantation of catheter and port system.
Statistical Analysis
Sensitivity and specificity were calculated for each arterial segment. Visualization scores between MSCTA and DSA were compared by two-sided Wilcoxon’s signed-rank test. The interobserver agreement for visualization score of MSCTA and DSA was evaluated using the κ value of two readers among four (six pairs each). Perfect agreement resulted in a κ value of 1; agreement expected on the basis of chance alone resulted in a κ value of 0. All calculations were performed using statistical analysis software (Dr. SPSS II 11.0.1.J; SPSS Inc., Chicago, IL).
Results
Patient Characteristics
Forty-six patients with unresectable malignant liver tumors, 29 males and 17 females, ranging in age from 36 to 83 years (median, 63 years), were enrolled in this study. Thirty-three patients had metastatic liver tumors, seven had hepatocellular carcinoma, three had gallbladder carcinoma, and three had cholangiocellular carcinoma.
Image Evaluation
In all patients, MSCTA examination was technically adequate and was suitable for analysis. In one patient, DSA was not performed, because MSCTA showed severe stenosis of the common celiac trunk and the superior mesenteric artery (i.e., celiacomesenteric trunk), and we judged that insertion of an angiographic catheter for DSA and placement of an arterial infusion catheter should be avoided to prevent catheter-related complications such as vascular injury and spasm resulting in possible hepatic or mesenteric ischemia, due to this anatomical variation. Therefore, 45 of 46 patients underwent DSA and entered in the analysis.
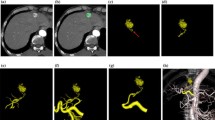
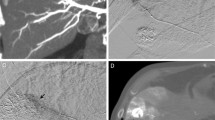
Anatomical variations of the hepatic artery were present in 19 of 45 patients (42%) on both MSCTA and DSA (Table 1, Figs. 1 and 2). The sensitivity of MSCTA was 100% and the specificity was 100% when DSA was used as the gold standard. On MSCTA and DSA, the course of the proximal portion of the celiac trunk was cranial in 9 patients on MSCTA/13 patients on DSA, horizontal in 7 on MSCTA/9 on DSA, and caudal in 29 on MSCTA and 23 on DSA. So, the courses of the celiac trunk in two modalities were not corresponding in 12 patients. Regarding the visualization scores of each arterial segment, no statistically significant differences were noted between MSCTA and DSA (Table 2, Figs. 3 and 4). κ values, which were used to assess interobserver agreement for visualization scores, ranged from 0.47 to 0.50 (mean, 0.49) for MSCTA and from 0.41 to 0.57 (mean, 0.48) for DSA. Stenosis of the celiac trunk was noted in two patients on both MSCTA and DSA (Fig. 5).
Technical Outcome
Except for one patient who did not undergo DSA and catheter placement due to MSCTA findings mentioned above, 45 patients in this study received interventional procedures—unification of multiple hepatic arteries, embolization of gastric arteries arising from hepatic arteries, and catheter placement—which were successful in all patients.
Estimated Radiation Dose
Calculated CTDIvol for this MSCTA acquisition protocol was 33.8 mGy. Mean DLP ± standard deviation was 773 ± 95 mGy.
Discussion
This prospective study shows that MSCTA provides important information for radiological catheter and port placement for HAIC. Anatomical variation of the hepatic artery was demonstrated in 50% of patients on MSCTA, with a sensitivity of 100% and a specificity of 100%. Visualization of each vascular segment showed no statistically significant differences between MSCTA and DSA. Interobserver agreement for visualization score of MSCTA was equivalent to that of DSA.
The hepatic vessels have a complicated anatomy, and we frequently face anatomical variants during the interventional procedure of hepatic arterial catheter placement. Such variants of the hepatic artery affect the technical outcome of our interventional procedure. If there are any replaced or accessory hepatic arteries, arterial redistribution to unify hepatic arteries is required to achieve the delivery of chemotherapeutic agents to the entire liver via an indwelling catheter [1, 3]. Recent studies have shown that MSCTA is accurate in assessing hepatic and gastric arteries [7–10]. The improvement in longitudinal resolution with MSCT is a substantial advantage over single helical or nonhelical CT [7–13]. The shorter acquisition time may also be advantageous for obtaining a pure arterial phase by eliminating contamination of venous or portal venous structures [13–14]. In previous studies, MSCTA demonstrated anatomical variation in 35% to 69% of patients [8, 9, 12, 14]. With regard to preoperative evaluation for surgical HAIC pump placement using laparotomy, two studies demonstrated an advantage of MSCTA [15, 16]. However, there is no report on CTA regarding radiological catheter and port placement. In radiological catheter and port placement, gastrointestinal arteries arising from hepatic arteries need to be embolized to avoid possible adverse gastrointestinal events due to exposure to high-dose chemotherapeutic agents [1, 3, 17]. Among the branches, the right gastric artery, arising from hepatic arteries, is relatively difficult to visualize on CTA because it tends to be small in diameter [10, 17]. Takahashi et al. reported the usefulness of MSCTA for depiction of small hepatic artery branches [10]. They reported that MSCTA detected 50 RGAs in 56 patients. The previous studies of preoperative assessment for HAIC pump under laparotomy did not include assessment of the gastroduodenal branches [15, 16]. In our study, mean visualization scores on MSCTA/DSA were 2.9 ± 0.2/2.9 ± 0.3 for GDA and 2.1 ± 0.8/2.2 ± 0.9 for RGA. Visualization scores for the RGA were lower than for other hepatic arterial segments in both modalities, probably because of its small size and complex course. However, the overall visualization score of MSCTA was equivalent to that of DSA. MSCTA is advantageous in giving information in advance of catheter and port placement, which may reduce the procedural time and avoid complications.
The course of the celiac axis also affects the technique of catheter placement. When a transfemoral or transepigastric approach is used, a caudal course of the celiac axis tends to be more difficult than a cranial course because multiple inflection points result in a reduction in torque of the catheter and guide wire. When the course is caudal, use of a specific J-type long sheath is considered for implantation of the catheter and port system [18]. Evaluation of the celiac axis prior to catheter and port implantation is important for planning the procedure. In this study, respiratory phase was consistent with routine examination and different between MSCTA and DSA. Lee et al. reported that there was no significant difference between inspiration and expiration in the angles between the celiac trunk and the aorta on MRI [19]. Factors other than respiration, such as redundant and tortuous celiac axis, may have affected the difference in our study. Even considering the effect of respiration, MSCTA showed an advantage in detecting the origin of the celiac trunk from the aorta constantly. Celiac DSA did not always demonstrate the origin of the celiac trunk. The 3-D capability of MSCTA is also effective for visualization of the celiac trunk from various directions.
Another important indication of preoperative vascular evaluation for HAIC is to detect vascular abnormalities such as stenosis or occlusion along the implantation route. Stenosis or occlusion may result not only in complications during procedures of angiography and catheter implantation, but also in early occlusion of the hepatic artery after catheter placement. In a postprocedure setting, MSCTA with intra-arterial administration of contrast material from an implanted catheter and port system help detect arterial stenosis [20]. In the present study, MSCTA with intravenous administration of contrast material demonstrated stenosis in two patients. In one patient with severe stenosis of the common stem of the celiac and mesenteric arteries, in which even diagnostic angiography poses a risk of complication, we could preclude angiography and catheter placement based on MSCTA findings.
In our series, interventional radiological placement of the catheter and port system was successful in all patients with preprocedural planning by MSCTA. Previously, we performed two sessions of DSA occasionally, one for evaluation of vascular anatomy and partial embolization and the other for placement of the catheter and port system and embolization. After implementation of MSCTA, this procedure has been done in one session, which may result in a shorter hospital stay.
Optimization of the radiation dose is crucial for MSCTA. In the current study, the radiation dose represented by CTDIvol and DLP was equivalent to the criteria from the European Guidelines on Quality Criteria for CT: weighted CTDI of 35 mGy and weighted DLP of 780 mGy · cm [21]. Although the radiation dose was not as low as in the previous report of a spiral and conventional CT study [22], our result is acceptable because this examination is designed for single, not repeated, preprocedural assessment including evaluation of liver tumor, nodal, and peritoneal tumor extent. Furthermore, MSCTA images provide adequate information on vascular mapping, and they lead to an overall reduction of radiation dose. Nonetheless, further radiation dose reduction should be made, while maintaining image quality. Techniques to reduce the radiation dose include reducing the milliampere-second value, increasing the pitch, adjusting the milliampere-second value according to the patient’s size, and reducing the beam energy [11, 23]. More important is to eliminate unnecessary CT examination or excessive multiphase study.
Limitations of our study include the small number of patients and lack of comparison with other noninvasive modalities such as duplex US. At our institute, contrast-enhanced CT is routinely used for pretreatment evaluation of malignant hepatic tumors and additional MSCTA in the same study was considered to be adequate. Another limitation is that we did not evaluate diverse reformation techniques for MSCTA, such as volume rendering (VR). We chose the MPVR technique based on our previous experience and reports by other investigators [9, 18, 24]. The application of target VR eliminated partial volume averaging on MPVR. This technique was useful in the visualization of small arteries such as the RGA, however, other reformation techniques should be taken into consideration.
In conclusion, MSCTA is accurate in the detection of abdominal arterial anatomy, variations, and abnormalities. MSCTA is suitable for planning of the catheter and port system implantation for HAIC.
References
Arai Y, Inaba Y, Takeuchi Y (1997) Interventional techniques for hepatic arterial infusion chemotherapy. In: Casterneda-Zuniga E (ed) Interventional radiology. Williams & Wilkins, Baltimore, pp 192–205
Kemeny NE, Ron IG (1999) Hepatic arterial chemotherapy in metastatic colorectal patients. Semin Oncol 26:524–535
Tanaka T, Arai Y, Inaba Y, et al. (2003) Radiologic placement of side-hole catheter with tip fixation for hepatic arterial infusion chemotherapy. J Vasc Interv Radiol 14:63–68
Meta-Analysis Group in Cancer (1996) Meta-Analysis Group in Cancer: reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. J Natl Cancer Inst 88:252–258
Kemeny N, Niedzwiecki D, Hollis DR (2006) Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 24:1395–1403
Seki H, Kimura M, Yoshimura N, et al. (1999) Hepatic arterial infusion chemotherapy using percutaneous catheter placement with an implantable port: assessment of factors affecting patency of the hepatic artery. Clin Radiol 54:221–227
Guiney MJ, Kruskal JB, Sosna J, et al. (2003) Multi-detector row CT of relevant vascular anatomy of the surgical plane in split-liver transplantation. Radiology 229:401–407
Sahani D, Saini S, Pena C, et al. (2002) Using multidetector CT for preoperative vascular evaluation of liver neoplasms: technique and results. AJR 179:53–59
Byun JH, Kim TK, Lee SS, et al. (2003) Evaluation of the hepatic artery in potential donors for living donor liver transplantation by computed tomography angiography using multidetector-row computed tomography: comparison of volume rendering and maximum intensity projection techniques. J Comput Assist Tomogr 27:125–131
Takahashi S, Murakami T, Takamura M, et al. (2002) Multi-detector row helical CT angiography of hepatic vessels: depiction with dual-arterial phase acquisition during single breath hold. Radiology 222:81–88
McNitt-Gray MF (2002) AAPM/RSNA physics tutorial for residents: topics in CT: radiation dose in CT. Radiographics 22:1541–1553
Erbay N, Raptopoulos V, Pomfret EA, et al. (2003) Living donor liver transplantation in adults: vascular variants important in surgical planning for donors and recipients. AJR 181:109–114
Fishman EK (2001) From the RSNA refresher courses: CT angiography: clinical applications in the abdomen. Radiographics 21:3–16
Winter TC III, Nghiem HV, Freeny PC, et al. (1995) Hepatic arterial anatomy: demonstration of normal supply and vascular variants with three-dimensional CT angiography. Radiographics 15:771–780
Sahani DV, Krishnamurthy SK, Kalva S, et al. (2004) Multidetector-row computed tomography angiography for planning intra-arterial chemotherapy pump placement in patients with colorectal metastases to the liver. J Comput Assist Tomogr 28:478–484
Kapoor V, Brancatelli G, Federle MP, et al. (2003) Multidetector CT arteriography with volumetric three-dimensional rendering to evaluate patients with metastatic colorectal disease for placement of a floxuridine infusion pump. AJR 181:455–463
Yamagami T, Nakamura T, Iida S, Kato T, Nishimura T (2002) Embolization of the right gastric artery before hepatic arterial infusion chemotherapy to prevent gastric mucosal lesions: approach through the hepatic artery versus the left gastric artery. AJR 179:1605–1610
Katoh K, Sone M, Nakasato T, et al. (2006) A new method using J-type long sheath for implantation of indwelling catheters for trans-femoral hepatic arterial infusion. Radiat Med 24:80–83
Lee VS, Morgan JN, Tan AGS, et al. (2003) Celiac artery compression by the median arcuate ligament: a pitfall of end-expiratory MR imaging. Radiology 228:437–442
Sone M, Kato K, Nakasato T, et al. (2002) Multislice CT angiography through an implantable catheter and port system: early experience in detection of vascular complications during hepatic arterial infusion chemotherapy. J Comput Assist Tomogr 26:515–519
European guidelines on quality criteria for computed tomography. Available at: http://www.drs.dk/guidelines/ct/quality/. Accessed December 12, 2006
Hidajat N, Wolf M, Nunnemann A, et al. (2001) Survey of conventional and spiral CT doses. Radiology 218:395–401
Karla MK, Maher MM, Toth TL, et al. (2004) Strategies for CT radiation dose optimization. Radiology 230:619–628
Mallouhi A, Rieger M, Czermak B, et al. (2003) Volume-rendered multidetector CT angiography: noninvasive follow-up of patients treated with renal artery stents. AJR 180:233–239
Acknowledgment
We are grateful to the Japanese Society of Implantable Port Assisted Regional Treatments (JSIPART) for awarding and supporting this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sone, M., Kato, K., Hirose, A. et al. Impact of Multislice CT Angiography on Planning of Radiological Catheter Placement for Hepatic Arterial Infusion Chemotherapy. Cardiovasc Intervent Radiol 31, 91–97 (2008). https://doi.org/10.1007/s00270-007-9170-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-007-9170-y