Abstract
The purpose of the study was to assess the effectiveness of contrast-enhanced ultrasonography (CEUS) in endoleak classification after endovascular treatment of an abdominal aortic aneurysm compared to computed tomography angiography (CTA). From May 2001 to April 2003, 10 patients with endoleaks already detected by CTA underwent CEUS with Sonovue® to confirm the CTA classification or to reclassify the endoleak. In three conflicting cases, the patients were also studied with conventional angiography. CEUS confirmed the CTA classification in seven cases (type II endoleaks). Two CTA type III endoleaks were classified as type II using CEUS and one CTA type II endoleak was classified as type I by CEUS. Regarding the cases with discordant classification, conventional angiography confirmed the ultrasound classification. Additionally, CEUS documented the origin of type II endoleaks in all cases. After CEUS reclassification of endoleaks, a significant change in patient management occurred in three cases. CEUS allows a better attribution of the origin of the endoleak, as it shows the flow in real time. CEUS is more specific than CTA in endoleak classification and gives more accurate information in therapeutic planning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endovascular aneurysm repair (EVAR) is a valid therapeutic alternative to traditional surgery in selected patients [1, 2]. Incomplete exclusion of the aneurysm sac—defined as an “endoleak”—is the most common complication after this procedure. An endoleak has an incidence rate of 10–45% [3]. It reveals itself with the presence of perigraft blood flow and sac revascularization, associated with a risk of subsequent rupture [4]. Endoleaks are classified according to their origin and risk of rupture. Type I endoleaks are due to inadequate proximal or distal anchoring; because there is no chance of spontaneous correction, they always require prompt intervention. Type II endoleaks—due to the presence of retrograde collateral flow (lumbar arteries, inferior mesenteric artery)—might resolve spontaneously and can thus be kept under control over time [5]. Type III endoleaks are caused by prosthetic fabric defects or graft detachment and also require prompt treatment. Type IV endoleaks are quite rare and are connected to graft porosity [6].
Because endoleaks could arise at any time after endograft deployment, long-term follow-up is required not only to detect any recurrence but also to classify them correctly.
The best postsurgery follow-up regime for patients undergoing EVAR is still disputed. The most used imaging technique is computed tomography angiography (CTA) [7], which has a sensitivity and specificity satisfactory for endoleak identification [8–10] although it seems less able to classify them [11]. The sources of endoleaks, however, are of great importance in therapeutic decisions. In addition, as CTA is expensive, requires contrast agents, and imposes a large radiation burden, alternative methods have been considered, especially ultrasound.
Some studies compared basal echo color Doppler and CTA in endoleak detection, with partly conflicting results [11–18]. The use of a first-generation contrast agent associated with echo color Doppler not only increases the method’s sensitivity, but it also raises the number of false positives, mainly due to artifacts [19–22]. The arrival of second-generation contrast media has enabled artifact-related problems to be overcome, allowing more accurate diagnosis of endoleaks [3, 23].
Nonetheless, few literature studies compared CTA and contrast enhanced ultrasound (CEUS) with respect to their ability to classify endoleaks correctly [3, 11, 24]. This article reports the results of a preliminary study on the use of CEUS with a second-generation contrast agent to reclassify 10 endoleaks already identified by CT.
Materials and Methods
Between May 2001 and April 2003, 120 patients underwent EVAR at our center. Among them, 10 patients (9 male, 1 female; mean age: 73.4 years, range: 53–79; mean aneurysm diameter 6.3 cm, range: 4.2–10 cm) who developed an endoleak documented by biphasic CTA after a time ranging from a few days to 6 months were selected to undergo CEUS to confirm the classification or to reclassify the endoleaks (Table 1).
Seven patients underwent scheduled treatment and three patients underwent emergency treatment (one for fissuration, two for rupture). Seven Excluder bifurcated endografts (Gore, Flagstaff, AZ, USA) and three Zenith bifurcated endografts with suprarenal anchoring (Cook, Bloomington, IN, USA) were used.
Computed tomography angiography was performed on a four-slice CT multidetector (Light Speed Plus; GE Medical Systems, Milwaukee, WI, USA) before and after intravenous injection of 120 mL of iodized contrast agent (Iomeron 300; Bracco, Milan, Italy) at a flow rate of 3 mL/sec with scans from 1 cm above the emergence of the celiac axis from the aorta to the femoral bifurcation. Thin-layer (2.5-mm) contrast sequences with reconstruction thickness of 1.2 mm were performed in the arterial phase using an automatic bolus time test (Smart Prep) and in the venous phase with a 80-sec delay after injection. Images were processed by dedicated software at an independent workstation to enable multiplanar, volume-rendering, and maximum intensity projection reconstructions. Parameters evaluated on CT scans were the leakage’s location, the endoleak position in relation to the endograft, the density of the endoleak, and patency of lumbar artery or inferior mesenteric artery [5, 6, 25].
Patients underwent echo color Doppler examinations using a 3–5-MHz probe, with longitudinal and transversal scans with the patient lying in a dorsal or lateral position. After the informed consent was obtained, they underwent B-mode CEUS with a 5-MHz probe and with a low Mechanical Index (varying from 0.093 to 0.157) real-time tissue harmonic imaging (Contrast Tuned Imaging CnTI) using Technos MPX ultrasound (ESAOTE Biomedica, Genoa, Italy) that enables selected tuning of the signal from the contrast agent microbubble resonance, notably filtering tissue echoes. A second-generation contrast agent (SonoVue; Bracco, Milan, Italy) consisting of sulfur hexafluoride gas microbubbles in a phospholipid membrane, which presents a longer persistence in the bloodstream under insonation by low acoustic power, was injected intravenously in all cases. Immediately after injection of 2.4 mL of the contrast agent as a single bolus, followed by 10 mL of saline, the chronometer and analysis archiving were started, so that the entire examination was tape-recorded to allow later review. The choice of contrast agent dose was justified by the need of ensuring visualization of the smallest endoleaks too. The entire abdominal aorta was examined up to the iliac branches by axial and longitudinal scans for 10 min after injection. Ultrasound was always performed by the same sonologist, blinded to CTA characterization. Images analysis at the time of ultrasound examinations assessed the presence of contrast enhancement within the aneurysm sac, with particular attention to the time of appearance (synchronous or delayed with respect to graft enhancement) and persistence (washout) and to inflow and outflow vessels.
In three cases, a digital subtraction angiography (DSA) study was also performed, using transfemoral percutaneous access after local anesthesia with 2% carbocaine. Aortography was performed with a 5F pigtail catheter positioned first above the proximal attachement site and then above the biforcation with injection of 40 mL of iodized contrast agent (Iomeron 300), obtaining anterior–posterior and lateral aortograms. In two cases, the exam was completed by a selective study of the internal iliac artery to confirm a type II endoleak originating from the right iliolumbar artery and a type I endoleak originating from the distal attachment site of the graft.
Results
Computed tomography angiography characterized eight endoleaks as type II and two as type III (Table 1). Echo color Doppler examinations revealed only seven endoleaks; in three patients, due to their physical constitution and metallic artefacts no identification was possible. Echo color Doppler identified one type II endoleak; it was inconclusive in the other six.
Contrast-enhanced ultrasound confirmed seven type II endoleaks, in agreement with CTA (70%); in contrast, it found one endoleak, classified as type II by CT, to be type I (Figs. 1A and 1B) and two type III endoleaks to be type II. The type I endoleak was synchronous to the appearance of contrast in the endoprosthesis, whereas in the type II endoleak, contrast enhancement appeared on average with 23–40 sec delay. CEUS then highlighted that perigraft supply of type II endoleaks came from the lumbar arteries or the inferior mesenteric artery (in one case, there was a double supply; Fig. 2), visualizing only inflow vessels.
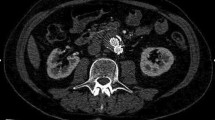
(A) Transverse CT angiogram: patent bifurcated aortic stent-graft (white arrow); contrast enhancement is seen inside the sac posterior to the patent limbs (black arrow) (characterized as a type II endoleak). (B) Cross-sectional contrast-enhanced ultrasound: flow inside the aneurysm sac coming from the distal stent attachment site. This finding is suggestive for a type I endoleak (white arrow). (C) Preprocedure DSA: extravasation of contrast material outside the graft depicting a type I endoleak (black arrow). (D) Postprocedure DSA: endoleak exclusion by deployment of an iliac extension graft.
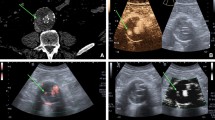
(A) Transverse CT angiogram: extravasation of contrast material within the native aorta evident anterior to the patent limbs of the stent-graft (white arrow); lighter contrast intensity is seen posteriorly (black arrow) (characterized as a lumbar type II endoleak). (B) Cross-sectional CEUS: the flow inside the aneurysmatic sac is coming from a reconstitution endoleak by the inferior mesenteric artery (inflow vessel) (a type II endoleak) (white arrow). (C) Cross-sectional CEUS: flow inside the sac coming from a lumbary artery (inflow vessel) (a type II endoleak) (white arrow). (D) Longitudinal-section CEUS: sac reconstitution from mesenteric and lumbary arteries are seen together (inflow vessels) (white arrows).
For the three cases of disagreement between CTA and CEUS, the ultrasound classification was confirmed by DSA (Fig. 1C). The CEUS classification changed the patient management in three cases: the type I endoleak was promptly corrected with placement of a right distal iliac extension graft (Fig. 1D). Two type II endoleaks healed spontaneously, with sac thrombosis at 6 month CTA follow-up. In the other seven cases, the mean CTA follow-up of 15 months (range: 12–24 months) showed the persistence of the endoleaks; in these patients, the aneurysm sac diameter remained unchanged and no other therapeutic measures were considered.
Discussion
The main purpose of postsurgery follow-up of patients after EVAR is to evaluate its technical and clinical success [26]. It is important to diagnose the appearance of any endoleak, as sac perfusion is a risk of rupture [27].
Biphasic CTA is considered the gold standard for follow-up analysis, as it reliably detects endoleaks. Precontrast and postcontrast arterial and venous phase scanning enables identification of both small endoleaks next to metallic parts or calcifications [10] and low-flow endoleaks visible in delayed scans [8]. However, CTA provides information on morphology and not on the direction of blood flow; thus, it is not always able to classify the origin of endoleaks correctly [28].
Ultrasound is an interesting alternative, and it costs less and causes less biological damage to patients who are not exposed to ionising radiation.
From a review of the literature it is found that basal color Doppler’s sensitivity (CDU) in identifying endoleaks ranges from 42% to 97%, with an overall specificity from 75% to 98.4% [11, 14–18]. The method’s limitations, regarding both sensitivity and specificity, are mainly due to echo reflection by the metallic part of the graft, extended calcifications, meteorism, obesity, and too-slow flow endoleaks; operator dependence should be added to these. Data concerning the sensitivity and specificity of CDU (versus CT) in classifying endoleaks are very limited [29] According to some authors [11, 14], CDU has the advantage over CTA to give hemodynamic information on blood flow and its direction: Thus, it is better than CTA for differentiating various endoleak types. The unsatisfactory CDU results shown in our study are due to operator difficulty in classifying them.
Echo amplifiers have been in use for several years, improving the image quality and highlighting even the weakest signals.
From some studies, it is deduced that echo color Doppler associated with a first-generation contrast agent has a sensitivity similar to that of CTA in endoleak identification [19, 20, 22]. However, examinations positive only with echo color Doppler cannot be considered as unequivocal, as they might be due to blooming artifacts enhanced by the contrast agent (false ultrasound positives) or to a CTA limitation in identifying slower-flow endoleaks (false CT negatives). Giannoni et al. [22], considering the absence of reduction—and sometimes the increase—in aneurysm diameter associated with such diagnoses, held that these are probably real type II endoleaks not seen with CTA and thus attributed greater sensitivity to CEUS. However, McWilliams et al. [21] called attention to the sensitivity limits of contrast-enhanced echo color Doppler, which in this study was 50% that of CTA, with a low negative predictive value (86%). The authors concluded that CTA remains the method of choice for follow-up.
The use of B-mode harmonic imaging with subtraction, associated with second-generation contrast media that contains more stable molecules and can therefore provide longer enhancement, seems a further evolution of ultrasound applications in the follow-up of patients after EVAR. These molecules generate harmonic signals that can be visualized even without color, with a consequent reduction in blooming artifacts.
Bendick et al. [3], in a study limited to 20 patients, identified 8 endoleaks revealed with biphasic CTA and B-mode harmonic imaging with subtraction associated with a second-generation contrast agent. In two other patients, a small proximal type I endoleak not seen at CTA was revealed by CEUS and subsequently confirmed by DSA: the presence of metallic artifacts near the graft attachment area probably prevented their visualization with CT. Furthermore, CEUS could classify eight endoleaks (type I or II), in contrast to CTA, which was inconclusive in three cases. Finally, CEUS facilitated evaluation of the distance between the graft’s proximal attachment and the renal artery origin [3].
In a recent study, Napoli et al. [23] evaluated the role of ultrasound with a second-generation contrast agent in a group of 10 patients, in whom basal echo color Doppler and biphasic CTA revealed an increased aneurysm diameter not accompanied by perigraft flow. CEUS identified the presence of an endoleak in all cases, confirmed by DSA in 8 out of 10 cases. The ultrasound examination found the aneurysm sac blood flow to be slow, delayed—only visible 2.5 min after contrast agent injection—and diffused within the sac. On the basis of the flow dynamics revealed by ultrasound, it can thus be hypothesized that the CTA scan performed later than routine (after 3–4 min) might increase CT sensitivity in detecting endoleaks. The reliability of CEUS is confirmed by the fact that in a second patient group, presenting reduced aneurysm sac volume and negative echo color Doppler and CTA examinations, the method did not give any false positives. Furthermore, in a third group with endoleaks already identified by other imaging methods, CEUS revealed no false negatives.
In our experience we wanted to evaluate CEUS ability in classifying endoleaks rather than studying CEUS sensibility in detection. In 10 harmonic ultrasound examinations with B-mode image subtraction using SonoVue, the method was able to confirm the origin of endoleaks visualized by CTA in seven cases (Type II) and correctly identifying the origin of the other three, in accordance with the angiographic study performed in the conflicting cases. Exact attribution of the endoleak origin enabled an interventional treatment in one case (type I) and a conservative treatment in the other two (type II).
Conclusions
In theory, CEUS is perhaps more reliable than CTA in studying endoleaks, as the relatively long time the contrast agent’s permanence in circulation (around 10 min) enables one to study patients also in a late phase. Furthermore, unlike CTA, this method is unaffected by metallic artifacts. CTA–apart from the problems connected with endoleak identification and classification–cannot currently be substituted, because it enables a more precise evaluation of the aneurysm sac diameter and provides information on graft anchoring, integrity, and morphology, unobtainable with ultrasound [26].
However, CEUS could be used along with CTA in cases where the latter reveals the presence of endoleaks, to give additional information on their classification. In fact, CEUS, taking advantage of the contrast agent’s angiodynamic behavior, permits an easier visualization while the agent flows into the aneurysm sac. It could also be indicated in patients with an aneurysm diameter increase in whom CTA did not reveal sac reperfusion [23]. Finally, type II endoleaks could be monitored with ultrasound, reducing the use of CTA with consequent reduction of costs and exposure to radiation.
Although these data are not enough to give sensitivity and specificity figures, they are encouraging. Further evaluations of the efficacy of CEUS with second-generation contrast agents must be performed with more cases before its routine use is proposed in the follow-up of patients after EVAR.
References
Parodi JC, Palmaz JC, Barone HD (1991) Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 5:491–499
Adriaensen ME, Bosch JL, Halpern EF, et al. (2002) Elective endovascular versus open surgical repair of abdominal aortic aneurysms: systematic review of short-term results. Radiology 24:739–747
Bendick PJ, Bove PG, Long GW, et al. (2003) Efficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts. J Vasc Surg 7:381–385
White RA, Donaayre C, Walot I, et al. (2000) Abdominal aortic aneurysm rupture following endoluminal graft deployment: Report of a predictable event. J Endovasc Ther 7:257–262
White GH, May J, Waugh RC, et al. (1998) Type I and II endoleaks: A more useful classification for reporting results of endoluminal AAA repair. J Endovasc Surg 5:189–191
White GH, May J, Waugh RC, et al. (1998) Type III and type IV endoleaks: Toward a complete definition of blood flow in the sac after endoluminal AAA repair. J Endovasc Surg 5:305–309
Karch LA, Henretta JP, Hodgson KJ (1999) Algorithm for the diagnosis and treatment of endoleaks. Am J Surg 178:225–231
Golzarian J, Dussaussois L, Abada HT (1998) Helical CT of aorta after endoluminal stent-graft therapy: value of biphasic acquisition. Am J Roentgenol 171:329–331
Gorich J, Rilinger N, Sokiranski R, et al. (1999) Leakages after endovascular repair of aortic aneurysms: Classification based on findings at CT, angiography, and radiography. Radiology 213:767–772
Rozenblit A, Patlas M, Rosenbaum A, et al. (2003) Detection of endoleaks after endovascular repair of abdominal aortic aneurysm: Value of unenhanced and delayed helical CT acquisitions. Radiology 227:426–433
Greenfield AL, Halpern EJ, Bonn J, et al. (2002) Application of duplex US for characterization of endoleaks in abdominal aortic stent grafts: Report of five cases. Radiology 225:845–851
McLafferty RB, McCrary BS, Mattos MA, et al. (2002) The use of colour-flow duplex scan for the detection of endoleaks. J Vasc Surg 36:100–104
Raman KG, Missing-Carroll N, Richardson T, et al. (2003) Flow-flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. J Vasc Surg 38:645–651
D’Audiffret A, Desgranges P, Kobeiter DH, et al. (2001) Follow-up evaluation of endoluminally treated abdominal aneurysms with duplex ultrasonography: validation with computed tomography. J Vasc Surg 33:42–50
Sato DT, Goff CD, Gregory RT, et al. (1998) Endoleak after aortic stent graft repair: Diagnosis by colour duplex ultrasound scan versus computed tomography scan. J Vasc Surg 28:657–663
Zannetti S, De Rango P, Parente B, et al. (2000) Role of duplex scan in endoleak detection after endoluminal abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 19:531–535
Pages S, Favre JP, Cerisier A, et al. (2001) Comparison of colour duplex ultrasound and computed tomography scan for surveillance after aortic endografting. Ann Vasc Surg 15:155–162
Wolf YG, Johnson BL, Hill BR, et al. (2000) Duplex ultrasound scanning versus computed tomographic angiography for postoperative evaluation of endovascular abdominal aortic aneurysm repair. J Vasc Surg 32:1142–1148
Heilberger P, Schunn C, Ritter W, et al. (1997) Postoperative colour flow duplex scanning in aortic endograft. J Endovasc Surg 4:262–271
McWilliams RG, Martin J, White D, et al. (1999) Use of contrast-enhanced ultrasound in follow-up after endovascular aortic aneurysm repair. J Vasc Intervent Radiol 10:1107–1114
McWilliams RG, Martin J, White D, et al. (2002) Detection of endoleak with enhanced ultrasound imaging: comparison with biphasic computed tomography. J Endovasc Ther 9:170–179
Giannoni MF, Palombo G, Sbarigia E, et al. (2003) Contrast-enhanced ultrasound imaging for aortic stent-graft surveillance. J Endovasc Ther 10:208–217
Napoli V, Bargellini I, Sardella S, et al. (2004) Abdominal aortic aneurysm: Contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology 233:217–225
Bargellini I, Napoli V, Petruzzi P, et al. (2005) Type II lumbar endoleaks: Hemodynamic differentiation by contrast enhanced ultrasound scanning and influence on aneurysm enlargement after endovascular aneurysm repair. J Vasc Surg 41:10–18
Stravropoulos W, Clark TWI, Carpenter JP, et al. (2005) Use of CT angiography to classify endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Intervent Radiol 16:663–667
White RA (2000) Endograft surveillance: a priority for long-term device performance [letter]. J Endovasc Ther 7:522
Harris PL, Vallabhanami SR, Desgranges P, et al. for the Eurostar Collaborators (2000) Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: The EUROSTAR experience. J Vasc Surg 32:739–749
Thurnher S, Cejna M (2002) Imaging of aortic stent-grafts and endoleaks. Radiol Clin N Am 40:799–833
Ashoke R, Brown L, Rodway A, et al. (2005) Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: A systematic review. J Endovasc Ther 12:297–305
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carrafiello, G., Laganà, D., Recaldini, C. et al. Comparison of Contrast-Enhanced Ultrasound and Computed Tomography in Classifying Endoleaks After Endovascular Treatment of Abdominal Aorta Aneurysms: Preliminary Experience. Cardiovasc Intervent Radiol 29, 969–974 (2006). https://doi.org/10.1007/s00270-005-0267-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-005-0267-x