Abstract
High-pressure and high-temperature Raman spectroscopic measurements of synthetic liebenbergite and Ni2SiO4 spinel have been conducted up to 22 GPa and 700 ℃, respectively. Isothermal and isobaric mode Grüneisen parameters were calculated based on the observed Raman modes. The intrinsic anharmonicities of liebenbergite and Ni2SiO4 spinel were also evaluated. The changes of the asymmetric SiO4 stretching band of Ni2SiO4 spinel in frequency are irreversible under decompression, indicating a potential pressure-induced modification in the crystal structure at elevated pressures. The values of isothermal mode Grüneisen parameters show that the SiO4 internal vibrations in Ni-rich olivines are more sensitive to the variations of pressure. For spinel-group minerals, the SiO4 internal vibrations can be less sensitive to the pressure change due to nickel incorporation. In contrast, according to the values of isobaric mode Grüneisen parameters, nickel increases the sensitivity of these vibrations to the variations of temperature. In addition, nickel has distinctive effects on the intrinsic anharmonicities of different vibration modes in both olivine and spinel-group minerals, and therefore alter the thermodynamic properties of their crystal structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The olivine (M2SiO4, M = divalent cation) orthosilicate and its high-pressure polymorphs are important phases of the Earth’s interior. In the upper mantle, (Mg, Fe)2SiO4 olivine (α-phase) is believed to be the most abundant mineral. It transforms to wadsleyite (β-phase) at 410-km depth and then to ringwoodite (γ-phase) at a depth of about 520 km in the mantle transition zone (Ringwood and Major 1970; Kohlstedt et al. 1996; Redfern et al. 2000; Chudinovskikh and Boehler 2001; Zhang and Smyth 2022). The olivine polymorphs can also be the major nickel reservoirs in the mantle (Ishimaru and Arai 2008; Straub et al. 2008; Zhang et al. 2018; Demouchy and Alard 2021; Zhang and Smyth 2022). In some localities such as Barberton, South Africa and Lavrion, Greece, Ni-dominant olivine (liebenbergite) and Ni-rich olivine do occur naturally (De Waal and Calk 1973; Tredoux et al. 1989; Koshlyakova et al. 2017). According to previous experimental investigations, pure liebenbergite transforms to Ni2SiO4 spinel at only 2–3 GPa (dependent on temperature) (Akimoto et al. 1965; Ma 1974). Therefore, the high-pressure and high-temperature behaviors of olivine polymorphs in Ni2SiO4 are expected to be significantly different from those in (Mg, Fe)2SiO4.
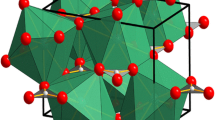
The crystal structure of liebenbergite consists of two symmetrically distinct NiO6 octahedral sites (M1 and M2) and one isolated SiO4 tetrahedral site (Si site). It is orthorhombic in the Pbnm space group. By contrast, Ni2SiO4 spinel is cubic in the Fd\(\:\stackrel{-}{3}\)m space group and contains only one octahedral site and one isolated tetrahedral site (Finger et al. 1979; Gartvich and Galkin 2019). The unit-cell parameters and crystal structures of Ni2SiO4 liebenbergite and spinel were previously measured at various pressure and temperature by the X-ray diffraction method (Mao et al. 1970; Sato 1977; Finger et al. 1979; Gartvich and Galkin 2019; Zhang et al. 2019). Based on the measurements under compression up to 12 and 30 GPa, the values KT (isothermal bulk modulus) of Ni2SiO4 spinel were calculated to be 214 and 223 GPa, respectively (Mao et al. 1970; Sato 1977). Finger et al. (1979) showed that, for Ni2SiO4 spinel, the polyhedral bulk modulus is about 170 GPa for the NiO6 octahedron and is greater than 250 GPa for the SiO4 tetrahedron. Zhang et al. (2019) found that liebenbergite can be the most incompressible olivine-group silicate. On the basis of the compressional equation of state measurement up to 42.6 GPa at room temperature, the value KT of liebenbergite was estimated to be about 163 GPa. Gartvich and Galkin (2019) determined temperature dependencies of molar volumes and coefficients of bulk thermal expansion of liebenbergite, and showed that the values of adiabatic bulk modulus KS and isothermal bulk modulus KT can be about 165 GPa and 163.7 GPa, respectively. For both Ni2SiO4 spinel and liebenbergite, according to Finger et al. (1979), the ratio of Ni-O distance in the octahedral site to Si-O distance in the tetrahedral site, (do/dt), decreases with increasing pressure or decreasing temperature.
Yamanaka and Ishii (1986) measured Raman spectra of Ni2SiO4 spinel in the temperature range of 20–600 ℃, and suggested that the Si-O bond can be significantly weakened at high temperatures. Lin (2001a) studied the Raman spectra of synthetic liebenbergite under compression up to 35 GPa in the quasi-hydrostatic (water was used as the pressure medium) and non-hydrostatic (no pressure medium) pressure environments. The results indicate that the distortion of SiO4 tetrahedra may be more severe than that of NiO6 octahedra at high pressures.
To better understand the high-pressure and high-temperature behaviors of liebenbergite and Ni2SiO4 spinel and the effects of nickel on the vibration properties of olivine and spinel-group minerals, we have synthesized liebenbergite and Ni2SiO4 spinel at 5 GPa, 1420 °C and 5 GPa, 1500 ℃, respectively. Tescan integrated mineral analyzer (TIMA) and single-crystal X-ray diffractometer were used to investigate their chemical compositions and crystal structures. In-situ high-pressure (argon was used as the pressure medium) and high-temperature Raman spectroscopic measurements of liebenbergite and Ni2SiO4 spinel have been conducted up to 22 GPa and 700 °C, respectively. Isothermal and isobaric mode Grüneisen parameters were calculated based on the experimental data. In addition, the intrinsic anharmonicities of liebenbergite and Ni2SiO4 spinel were also evaluated.
Experimental work
The liebenbergite and Ni2SiO4 spinel samples were respectively synthesized at 5 GPa, 1500 °C and 5 GPa, 1420 °C with heating time of 8 h in the GY-420 cubic-anvil apparatus at Center for High Pressure Science and Technology Advanced Research, Beijing. Pressure was calibrated based on Bi I-II and quartz-coesite phase transition at 2.55 GPa, room temperature and 3.2 GPa, 1100 °C, respectively. The temperature effect during the calibration process was not considered. High-purity oxide powders (NiO and SiO2) were mixed using ethanol in an agate mortar for 1–2 h and dried at 110 °C overnight. The mixed starting material powder was then filled into a Pt capsule (ID 3.2 mm, OD 3.5 mm, length 5 mm). To minimize thermal gradient, the capsule was put at the center of a graphite heater. During the experiments, the starting materials were cold pressed to target pressure and then heated to target temperature with a rate of 100 °C/min. Temperature was monitored using a W3Re-W25Re (type D) thermocouple controller. The runs were quenched by cutting the power and temperature (typically dropped down to 100 °C in < 50 s). The recovered capsules were mounted in epoxy and polished to expose the polycrystalline specimens.
For phase identification, the polished capsules were scanned by a TIMA3X FEG LMH analyzer with 8.83 nA probe current and 25,000 eV beam energy. The working distance, spot size, and pixel size were 15 mm, 91.67 nm, and 1 μm, respectively. The representative SEM images of the exposed run products are shown in Fig. 1. For crystal structure analysis, single crystals of liebenbergite and Ni2SiO4 spinel were selected from the run products, and then analyzed by using a Bruker D8 Venture X-ray diffractometer with a PHOTON detector. The X-ray (λ = 0.71073 Å) was generated at 50 kV and 1.4 mA via a IµS 3.0 generator equipped with a Mo anode. The crystal structures were refined from the collected intensity data with SHELX (Sheldrick 2015) in WINGX software (Farrugia 2012). The refinements were based on the reported scattering factors and absorption coefficients for Ni, O and Si in International Tables for Crystallography, Volume C (Prince 2004). The unit-cell and atom position parameters (Table S1) are estimated based on the results of single-crystal X-ray diffraction analyses. The X-ray powder diffraction patterns for Cu Kα radiation (λ = 1.5405 Å) of liebenbergite and Ni2SiO4 spinel were calculated using Vesta software (Fig. S1). As shown in Fig. 1, most of the starting composition successfully transformed to liebenbergite and Ni2SiO4 spinel, while minor residual unreacted NiO and SiO2 remain as bunsenite and coesite.
In the high-pressure Raman spectroscopic measurements, a pair of type Ia diamond anvils with 300 μm culet diameter was used to compress the sample in a symmetric-type diamond-anvil cell (DAC). The unoriented chips of liebenbergite (about 50 × 50 × 30 μm in size) and Ni2SiO4 spinel (about 40 × 30 × 30 μm in size) were loaded separately into the hole (approximately 150 μm in diameter and 40–50 μm in depth) in a rhenium gasket with one or two ruby spheres. Argon (Ar) was used as the pressure-transmitting medium. According to Klotz et al. (2009), the potential pressure gradients are expected to be lower than 0.2 GPa at pressures up to 20 GPa. Due to the rapid increase in pressure gradients over 20 GPa, the spectra obtained below 20 GPa were used to analyze the variations in Raman modes under compression and decompression. Raman spectra were collected by a HORIBA LabRAM HR Evolution laser Raman spectrometer with a 20×microscope objective at Institute of Geology, Chinese Academy of Geological Sciences. All spectra were excited by a 532 nm solid-state laser and collected in the wavenumber range from 100 to 1200 cm− 1, using 10 accumulations and 5 s exposure time with a spectral resolution of 1 cm− 1. A laser power of 40 mW was used at the surface of the sample. Pressures were estimated based on the shift of the ruby R1 luminescent line (Chijioke et al. 2005).
Raman spectra of the unoriented liebenbergite (about 50 × 40 × 40 μm in size) and Ni2SiO4 spinel (about 40 × 30 × 30 μm in size) at various temperature were analyzed by a WITec’s confocal Raman imaging microscope alpha300 R with a 20 × objective at Institute of Geology and Geophysics, Chinese Academy of Sciences. A Linkam TS1400 heating stage with a SiO2 window and a Rh/Pt thermocouple was used for the high-temperature measurements at 25, 100, 200, 300, 400, 500, 600, and 700 °C during heating and cooling (increasing and decreasing rates are 50 °C/min and − 50 °C/min, respectively). At each temperature, the sample was held for 10 min to reach equilibrium. All spectra were excited by a 488-nm solid-state laser and collected in the wavenumber range of 200–1000 cm− 1 with 40 mW power on the sample, using 1800 gr/mm grating with 15 accumulations and 20 s exposure time.
Results and discussion
Raman spectra of liebenbergite and Ni2SiO4 spinel at ambient conditions
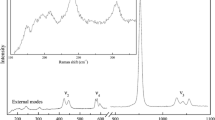
As summarized in Fig. 2, the unpolarized Raman spectrum of liebenbergite displays 15 bands at 221, 254, 272, 294, 300, 305, 347, 425, 560, 567, 819, 840, 867, 890, and 954 cm− 1 at ambient conditions. All these bands are in agreement with those observed in previous studies (Lin 2001a, b). The bands at 221, 254, 294, 300, and 305 cm− 1 are assigned to the M2 and SiO4 translations (Ag). The bands at 272 (B2g) and 347 cm− 1 (B3g) are due to SiO4 rotations. The 425 cm− 1 band and the two bands at about 560 cm− 1 correspond to the symmetric (ν2) and asymmetric (ν4) deformation of the SiO4 tetrahedron, respectively. The last four bands (at 840, 867, 890, and 954 cm− 1) and the intense band at 819 cm− 1 are assigned to the asymmetric (ν3) and symmetric (ν1) stretching of the SiO4, respectively (Lin 2001a, b).
Under ambient conditions, the unpolarized Raman spectrum of Ni2SiO4 spinel contains three strong bands at 371, 812 and 850 cm− 1 (Fig. 2). The latter two bands at 812 and 850 cm− 1 are assigned to the asymmetric (T2g) and symmetric (A1g) stretching of the SiO4 tetrahedron, respectively (Chopelas et al. 1994; Kleppe et al. 2002b), while the band at 371 cm− 1 corresponds to the deformation (Eg) of the SiO4. The weak band at 712 cm− 1 is typically assigned to the SiO4 internal vibrations in crystal structures of olivine and spinel-group minerals (Yamanaka and Ishii 1986; Chopelas et al. 1994; Yang et al. 2015; Liu et al. 2021). In hydrous Mg-Fe ringwoodite, according to Kleppe et al. (2002a), this band can be due to stretching of Si2O7 dimer, which is incompatible with the ideal spinel structure (contains only isolated SiO4 tetrahedra). The presence of the dimer with a bridging oxygen in the spinel structure, would require a non-silicate oxygen elsewhere which could act as site for protonation (Kleppe et al. 2002a). Therefore, Si2O7 groups are not expected to exist in the crystal structure of the anhydrous Ni2SiO4 spinel in this study.
Raman spectra of liebenbergite and Ni2SiO4 spinel as a function of pressure
The representative Raman spectra of liebenbergite and Ni2SiO4 spinel as a function of increasing and decreasing pressure are shown in Figs. 3 and 4, respectively. For liebenbergite, the three bands (at 560, 819, and 840 cm− 1 under ambient pressure) caused by the stretching and deformation of the SiO4 shift continuously to higher frequencies with increasing pressure up to 22.54 GPa. As shown in Fig. 5, these spectral changes are reversible on decompression. The intensities of the other eight bands observed at ambient conditions decrease progressively during compression. As a result, these bands gradually become indistinguishable in the pressure range of 1.36–15.98 GPa. However, they recover in the identical range with decreasing pressure (Table S2). In addition, no new modes can be observed in all the spectra during compression and decompression (Fig. 3). Thus, liebenbergite is not expected to undergo remarkable changes in crystal structure within the studied pressure range.
For Ni2SiO4 spinel, the three bands associated with the stretching and deformation of the SiO4 shift continuously up to 21.70 GPa under compression. The weak band at 712 cm− 1 is difficult to be observed due to its low intensity (Fig. 4). The two intense SiO4 stretching bands (at 812 and 850 cm− 1 under ambient pressure) of Ni2SiO4 spinel shift faster in frequency with increasing pressure than those (at 819 and 840 cm− 1 under ambient pressure) of liebenbergite (3.20–3.39 cm− 1/GPa compared to 3.03–3.14 cm− 1/GPa) (Table 1). The frequencies of the asymmetric SiO4 stretching band (at 812 cm− 1 under ambient pressure) can be strongly affected by the unit-cell parameters and increase nearly linearly with decreasing cell volume (Chopelas et al. 1994). As presented in Fig. 5 and Table S3, the frequency changes of this band are irreversible under decompression, indicating a pressure-induced modification in the crystal structure. According to Kamb (1968), the stability limit of Ni2SiO4 spinel can be simply defined by the ratio (do/dt) of octahedral (Ni-O) to tetrahedral (Si-O) distance. During compression, the do/dt ratio decreases with decreasing cell volume (Finger et al. 1979). Meanwhile, the stability of the crystal structure can be reinforced (Kamb 1968). Therefore, the modification may easily be retained under decompression. In-situ high-pressure X-ray diffraction investigations (on compression and decompression) are expected to further explain the irreversible frequency changes observed in this study.
Raman spectra of liebenbergite and Ni2SiO4 spinel as a function of temperature
The Raman spectra of liebenbergite and Ni2SiO4 spinel as a function of increasing and decreasing temperature in the range of 25–700 °C are shown in Figs. 6 and 7, respectively. For liebenbergite, the bands observed at ambient temperature shift continuously to lower frequencies with increasing temperature up to 700 °C (Figs. 6 and 8). In all the spectra collected during heating and cooling, no new bands and no evidence for temperature-induced amorphization can be observed. As shown in Fig. 8 and Table S4, all the bands return to their initial frequencies at ambient temperature after cooling, indicating no irreversible modifications of the crystal structure within the studied temperature range.
The Raman bands of Ni2SiO4 spinel also exhibit shifts to lower wavenumber upon heating to 700 °C (Figs. 7 and 8). The vibrational bands of the SiO4 (at 371, 812, 850 cm− 1 under ambient temperature) and their shifts are similar to those reported in the previous high-temperature (up to 600 °C) Raman study (Yamanaka and Ishii 1986). As shown in Fig. 8 and Table S5, the frequencies of the bands are fully reversible upon cooling. In addition, no bands disappear and no new bands emerge during heating and cooling. These results show no remarkable modifications of the Ni2SiO4 spinel sample in the investigated temperature range. During heating, the do/dt ratio increases with increasing cell volume (Finger et al. 1979), resulting in a reduced stability of the crystal structure (Kamb 1968). Thus, the potential modification can hardly be retained upon cooling.
As shown in Table 1, the strong band (at 850 cm− 1 under ambient temperature) associated with the symmetric SiO4 stretching of Ni2SiO4 spinel shifts slower in frequency than that (at 840 cm− 1 under ambient temperature) of liebenbergite upon heating (-0.014 cm− 1/°C compared to -0.018 cm− 1/°C). In contrast, the asymmetric stretching band of the SiO4 (at 812 cm− 1 under ambient temperature) of Ni2SiO4 spinel shifts significantly faster than that (at 819 cm− 1 under ambient temperature) of liebenbergite (-0.034 cm− 1/°C compared to -0.006 cm− 1/°C). Based on the previous investigation (Chopelas et al. 1994), the unit cell of Ni2SiO4 spinel is therefore believed to vary more rapidly in size with temperature.
Grüneisen parameters and anharmonicity
The isothermal mode Grüneisen parameter γiT and the isobaric mode Grüneisen parameter γiP represent the pressure and temperature dependence of the ith vibration mode at a constant temperature and constant pressure, respectively (Yang et al. 2017; Zhai et al. 2020).
The isothermal mode Grüneisen parameter γiT is calculated using the equation:
The isobaric mode Grüneisen parameter γiP is calculated using the equation:
Where vi is the Raman band of the ith vibrational mode at ambient temperature, \({\left( {{{\partial {v_i}} \over {\partial P}}} \right)_T}\) and \({\left( {{{\partial {v_i}} \over {\partial T}}} \right)_P}\) are the temperature and pressure derivatives of the Raman frequency obtained from the compression and heating processes in this study, KT is the isothermal bulk modulus at ambient temperature and α is thermal expansion coefficient.
The isothermal bulk modulus \(\:{K}_{T}=\) 163 GPa for liebenbergite (Zhang et al. 2019) and \(\:{K}_{T}=\) 226 GPa for Ni2SiO4 spinel (Bass et al. 1984) were used in the γiT calculation. To simplify the calculation (Liu et al. 2019), the average volume thermal expansion coefficients of 2.76 × 10−5 K−1 for liebenbergite (Kroll et al. 2019), 2.69 × 10− 5 K− 1 for Ni2SiO4 spinel (Finger et al. 1979) and 3.08 × 10− 5 K− 1 for ringwoodite (Katsura et al. 2004) were used in the γiP calculation.
As presented in Table 1; Figs. 9 and 10, the lattice vibration modes have higher γiT and γiP values than the SiO4 internal vibration modes in the crystal structures of both liebenbergite and forsterite. This indicates that the lattice vibrations are generally more sensitive to the variations of pressure and temperature compared to the SiO4 internal vibrations in olivines that contain metal cations with different radii.
Isobaric mode Grüneisen parameters for various Raman mode frequencies in forsterite (Gillet et al. 1991), ringwoodite, liebenbergite and Ni2SiO4 spinel. The isobaric mode Grüneisen parameter of ringwoodite was recalculated based on the temperature dependence reported in Liu and Mernagh (1994) and Liu et al. (2002)
The SiO4 internal vibration modes (at 560, 819 and 840 cm− 1) of liebenbergite have higher γiT values than those of forsterite. However, the SiO4 internal vibration modes (at 812 and 850 cm− 1) of Ni2SiO4 spinel have lower γiT values than those of ringwoodite (Table 1; Fig. 9). Therefore, nickel in olivine structure is believed to increase the sensitivity of the SiO4 internal vibrations to the variations of pressure. By comparison, these vibrations in spinel structure can be less sensitive to the pressure change due to nickel incorporation. As shown in Table 1; Fig. 10, the γiP values of the SiO4 internal vibration modes for Ni2SiO4 spinel are significantly higher than those for ringwoodite, revealing that nickel in the spinel structure increases the sensitivity of these vibrations to the variations of temperature.
The intrinsic anharmonic mode parameter ai is calculated based on the following equation:
The non-zero ai values (Table 1; Fig. 11) indicate that intrinsic anharmonicity exists in both liebenbergite and Ni2SiO4 spinel. According to Gillet et al. (1991), the ai values are negative for all vibration modes in forsterite (Fig. 11). However, in liebenbergite, the ai values for the lattice vibration mode at 272 cm− 1 and the SiO4 internal vibration mode at 819 cm− 1 are positive, though the values for the modes at 560 and 840 cm− 1 are also negative (Fig. 11). In ringwoodite, the ai values for the SiO4 internal vibration modes are positive. Whereas, the value for the asymmetric SiO4 stretching mode (at 812 cm− 1) in Ni2SiO4 spinel is negative, though the value for the symmetric SiO4 stretching mode (at 850 cm− 1) is also positive. These imply that nickel in olivine and spinel structures has distinctive effects on the intrinsic anharmonicities of different vibration modes.
Nickel is the fifth most abundant element in the Earth. The estimated concentrations of this element in the core, mantle, and bulk Earth are respectively 5.2 wt%, 0.2 wt%, and 1.82 wt% (McDonough 2014). However, the estimation of nickel concentration in the mantle is based heavily on the chemical compositions of rocks in the shallow upper mantle (Matzen et al. 2013; McDonough 2014; Palme and O’Neill 2014). The minerals in these rocks are too far removed from their mantle source to provide information on the enrichment of nickel in the interior of the silicate Earth (Matzen et al. 2013; Pu et al. 2017; Putirka et al. 2018). It is possible that nickel can be enriched in the olivine polymorphs in the deep part of the mantle under specific geological settings (Matzen et al. 2013; Zhang et al. 2019; Zhang and Smyth 2022). This study indicates that Ni incorporation can influence the thermodynamic properties of olivine and spinel group minerals by altering Grüneisen parameters of vibration modes and intrinsic anharmonicities. Since the mode Grüneisen parameters were calculated only based on Raman active optical phonons in the present work, more experimental and theoretical investigations are required to further reveal the Grüneisen parameters of phonon modes across the entire Brillouin zone.
Conclusions
-
(1)
For Ni2SiO4 spinel, the changes of the asymmetric SiO4 stretching band in frequency are irreversible during decompression, indicating a potential pressure-induced modification in the crystal structure at elevated pressures up to 22 GPa.
-
(2)
Nickel in olivine structure increases the sensitivity of the SiO4 internal vibrations to the variations of pressure. Due to nickel incorporation, the SiO4 internal vibrations in spinel structure can be less sensitive to the pressure change. However, these vibrations are more sensitive to the variations of temperature.
-
(3)
Nickel has distinctive effects on the intrinsic anharmonicity of different vibration modes in olivine and spinel structures.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Akimoto SI, Fujisawa H, Katsura T (1965) The olivine-spinel transition in Fe2SiO4 and Ni2SiO4. J Geophys Res 70(8):1969–1977. https://doi.org/10.1029/JZ070i008p01969
Bass JD, Weidner DJ, Hamaya N, Ozima M, Akimoto S (1984) Elasticity of the olivine and spinel polymorphs of Ni2SiO4. Phys Chem Min 10:261–272. https://doi.org/10.1007/BF00311951
Chijioke AJ, Nellis WJ, Soldatov A, Silvera IF (2005) The ruby pressure standard to 150 GPa. J Appl Phys 98(11):114905. https://doi.org/10.1063/1.2135877
Chopelas A (1990) Thermal properties of forsterite at mantle pressures derived from vibrational spectroscopy. Phys Chem Min 17:149–156. https://doi.org/10.1007/BF00199666
Chopelas A, Boehler R, Ko T (1994) Thermodynamics and behavior of γ-Mg2SiO4 at high pressure: implications for Mg2SiO4 phase equilibrium. Phys Chem Min 21:351–359. https://doi.org/10.1029/GL010i001p00087
Chudinovskikh L, Boehler R (2001) High-pressure polymorphs of olivine and the 660-km seismic discontinuity. Nature 411:574–577. https://doi.org/10.1038/35079060
De Waal SA, Calk LC (1973) Nickel minerals from Barberton, South Africa: VI. Liebenbergite, a nickel olivine. Am Min 58(7–8):733–735
Demouchy S, Alard O (2021) Hydrogen, trace, and ultra-trace element distribution in natural olivines. Phys Chem Min 176:26. https://doi.org/10.1007/s00410-021-01778-5
Farrugia LJ (2012) WinGX and ORTEP for Windows: an update. J Appl Crystallogr 45(4):849–854. https://doi.org/10.1107/S0021889812029111
Finger LW, Hazen RM, Yagi T (1979) Crystal structures and electron densities of nickel and iron silicate spinels at elevated temperature or pressure. Am Min 64(9–10):1002–1009
Gartvich Y, Galkin V (2019) Ni olivine: thermal behavior of liebenbergite. J Therm Anal Calorim 136:2333–2339. https://doi.org/10.1007/s10973-018-7859-6
Gillet P, Richet P, Guyot F, Fiquet G (1991) High-temperature thermodynamic properties of forsterite. J Geophys Res 96(B7):11805–11816. https://doi.org/10.1029/91JB00680
Ishimaru S, Arai S (2008) Nickel enrichment in mantle olivine beneath a volcanic front. Contrib Mineral Petr 156:119–131. https://doi.org/10.1007/s00410-007-0277-6
Kamb B (1968) Structural basis of the olivine-spinel stability relation. Am Min 53(9–10):1439–1455
Katsura T, Yokoshi S, Song M, Kawabe K, Tsujimura T, Kubo A, Ito E, Tange Y, Tomioka N, Saito K, Nozawa A, Funakoshi K (2004) Thermal expansion of Mg2SiO4 ringwoodite at high pressures. J Geophys Res 109:B12209. https://doi.org/10.1029/2004JB003094
Kleppe AK, Jephcoat AP, Smyth JR (2002a) Raman spectroscopic study of hydrous γ-Mg2SiO4 to 56.5 GPa. Phys Chem Min 29:473–476
Kleppe AK, Jephcoat AP, Smyth JR, Frost DJ (2002b) On protons, iron and the high-pressure behavior of ringwoodite. Geophys Res Lett 29(21):17. https://doi.org/10.1029/2002GL015276. -1-17-4
Klotz S, Chervin JC, Munsch P, Le Marchand G (2009) Hydrostatic limits of 11 pressure transmitting media. J Phys D: Appl Phys 42(7):075413. http://iopscience.iop.org/0022-3727/42/7/075413
Kohlstedt DL, Keppler H, Rubie DC (1996) Solubility of water in the α, β, and γ phases of (mg,Fe)2SiO4. Contrib Mineral Petr 123:345–357. https://doi.org/10.1007/s004100050161
Koshlyakova NN, Zubkova NV, Pekov IV et al (2017) Crystal chemistry of vanadate garnets from old metallurgical slags Lavrion, Greece. J Min Geochem 194:19–25. https://doi.org/10.1127/njma/2016/0010
Kroll H, Schmid-Beurmann P, Sell A, Büscher J, Dohr R, Kirfel A (2019) Thermal expansion and thermal pressure in Co and Ni olivines: a comparison with Mn and Fe olivines. Eur J Mineral 31:313–324. https://doi.org/10.1127/ejm/2019/0031-2805
Lin CC (2001a) High-pressure Raman spectroscopic study of Co- and Ni-olivines. Phys Chem Min 28:249–257. https://doi.org/10.1007/s002690100158
Lin CC (2001b) Vibrational spectroscopic study of the system α-Co2SiO4-α-Ni2SiO4. J Sol State Chem 157(1):102–109. https://doi.org/10.1006/jssc.2000.9044
Liu L, Mernagh TP (1994) Raman Spectra of high-pressure polymorphs of Mg2SiO4 at various temperatures. High Temp-High Press 26:631–637
Liu L, Lin CC, Mernagh TP, Inoue T (2002) Raman spectra of hydrous γ -Mg2SiO4 at various pressures and temperatures. Phys Chem Min 29:181–187. https://doi.org/10.1007/s00269-001-0219-1
Liu D, Pang Y, Ye Y, Jin Z, Symth JR, Yang Y, Zhang Z, Wang Z (2019) In-situ high-temperature vibrational spectra for synthetic and natural clinohumite: implications for dense hydrous magnesium silicates in subduction zones. Am Min 104(1):53–63. https://doi.org/10.2138/am-2019-6604
Liu D, Guo X, Symth JR, Wang X, Zhu X, Miao Y, Bai J, Ye Y (2021) High-temperature and high-pressure Raman spectra of Fo89Fa11 and Fo58Fa42 olivines: iron effect on thermodynamic properties. Am Min 106(10):1668–1678. https://doi.org/10.2138/am-2021-7686
Ma CB (1974) Reinvestigation of the olivine-spinel transformation in Ni2SiO4 and the incongruent melting of Ni2SiO4 olivine. J Geophys Res 79(23):3321–3324. https://doi.org/10.1029/JB079i023p03321
Mao HK, Takahashi T, Bassett WA (1970) Isothermal compression of the spinel phase of Ni2SiO4 up to 300 kilobars at room temperature. Phys Earth Planet Inter 3:51–53. https://doi.org/10.1016/0031-9201(70)90043-9
Matzen AK, Baker MB, Beckett JR, Stolper EM (2013) The temperature and pressure dependence of nickel partitioning between olivine and silicate melt. J Petrol 54(12):2521–2545. https://doi.org/10.1093/petrology/egt055
McDonough WF (2014) Compositional model for the earth’s core. In: Holland HD, Turekian KK (eds) Treatise on Geochemistry, 2nd edn. Elsevier, Oxford, pp 559–577. https://doi.org/10.1016/B978-0-08-095975-7.00215-1.
McKeown DA, Bell MI, Caracas R (2010) Theoretical determination of the Raman spectra of single-crystal forsterite (Mg2SiO4). Am Min 95(7):980–986. https://doi.org/10.2138/am.2010.3423
Palme H, O’Neill H (2014) Cosmochemical estimates of mantle composition. In: Holland HD, Turekian KK (eds) Treatise on Geochemistry, 2nd edn. Elsevier, Oxford, pp 1–39. https://doi.org/10.1016/B978-0-08-095975-7.00201-1
Prince E (2004) In: Prince E (ed) International tables for crystallography, volume C: mathematical physical and chemical tables, 3rd edn. Springer, Dordrecht, the Netherlands. https://doi.org/10.1107/97809553602060000103
Pu X, Lange RA, Moore G (2017) A comparison of olivine-melt thermometers based on DMg and DNi: the effects of melt composition, temperature, and pressure with applications to MORBs and hydrous arc basalts. Am Min 102(4):750–765. https://doi.org/10.2138/am-2017-5879
Putirka K, Tao Y, Hari KR, Perfit MR, Jackson MG, Arevalo R (2018) The mantle source of thermal plumes: Trace and minor elements in olivine and major oxides of primitive liquids (and why the olivine compositions don’t matter). Am Min 103(8):1253–1270. https://doi.org/10.2138/am-2018-6192
Redfern S, Artioli G, Rinaldi R, Henderson CMB, Knight KS, Wood BJ (2000) Octahedral cation ordering in olivine at high temperature. II: an in situ neutron powder diffraction study on synthetic MgFeSiO4 (Fa50). Phys Chem Min 27:630–637. https://doi.org/10.1007/s002690000109
Ringwood AE, Major A (1970) The system Mg2SiO4-Fe2SiO4 at high pressures and temperatures. Phys Earth Planet Inter 3:89–108. https://doi.org/10.1016/0031-9201(70)90046-4
Sato Y (1977) Equation of state of mantle minerals determined through high-pressure X-ray study. High Press Res 307–323. https://doi.org/10.1016/B978-0-12-468750-9.50028-0
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C 71(1):3–8. https://doi.org/10.1107/S2053229614024218
Straub SM, LaGatta AB, Pozzo ALMD, Langmuir CH (2008) Evidence from high-Ni olivines for a hybridized peridotite/pyroxenite source for orogenic andesites from the central Mexican Volcanic Belt. Geochem Geophys Geosyst 9(3):Q03007. https://doi.org/10.1029/2007GC001583
Tredoux M, de Wit MJ, Hart RJ, Armstrong RA, Lindsay NM, Sellschop JPF (1989) Platinum group elements in a 3.5 Ga nickel-iron occurrence: possible evidence of a deep mantle origin. J Geophys Res 94(B1):795–813. https://doi.org/10.1029/JB094iB01p00795
Yamanaka T, Ishii M (1986) Raman scattering and lattice vibrations of Ni2SiO4 spinel at elevated temperature. Phys Chem Min 13:156–160. https://doi.org/10.1007/BF00308157
Yang Y, Wang Z, Smyth JR, Liu J, Xia Q (2015) Water effects on the anharmonic properties of forsterite. Am Min 100(10):2185–2190. https://doi.org/10.2138/am-2015-5241
Yang M, Cheng X, Li Y, Ren Y, Liu M, Qi Z (2017) Anharmonicity of monolayer MoS2, MoSe2, and WSe2: a Raman study under high pressure and elevated temperature. Appl Phys Lett 110(9):093108. https://doi.org/10.1063/1.4977877
Zhai K, Xue W, Wang H, Wu X, Zhai S (2020) Raman spectra of sillimanite, andalusite, and kyanite at various temperatures. Phys Chem Min 47:23. https://doi.org/10.1007/s00269-020-01092-9
Zhang L, Smyth JR (2022) Crystal chemistry of metal element substitution in olivine and its high-pressure polymorphs: implications for the upper-mantle and the mantle transition zone. Earth-Sci Rev 232:104127. https://doi.org/10.1016/j.earscirev.2022.104127
Zhang L, Smyth JR, Kawazoe T, Jacobsen SD, Qin S (2018) Transition metals in the transition zone: partitioning of Ni, Co, and zn between olivine, wadsleyite, ringwoodite, and clinoenstatite. Contrib Mineral Petr 173:52. https://doi.org/10.1007/s00410-018-1478-x
Zhang D, Hu Y, Xu J, Downs RT, Hammer JE, Dera PK (2019) High-pressure behavior of liebenbergite: the most incompressible olivine-structured silicate. Am Min 104(4):580–587. https://doi.org/10.2138/am-2019-6680
Funding
This study was supported by the National Natural Science Foundation of China (No. 42172044 to L.Z. and 42250105 to Y.L.) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
L.Z. suggested the basis of the paper; L.Z. and S.G. wrote the main manuscript text; S.G. and H.C. performed high-pressure and high-temperature experiments; J.L. performed TIMA analysis; S.G. and X.L. performed high-temperature Raman measurements; S.G, J.L., and X.Q. performed high-pressure Raman measurements. L.Z. and Y.L. suggested and discussed the methods and results; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, S., Liu, J., Cheng, H. et al. Raman spectroscopic study of liebenbergite and Ni2SiO4 spinel at high pressure and high temperature: nickel effects on the vibration properties of olivine and spinel structures. Phys Chem Minerals 51, 35 (2024). https://doi.org/10.1007/s00269-024-01295-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-024-01295-4