Abstract
Phase transformations of the charoite mineral induced by thermal treatment at high temperatures were studied by simultaneous monitoring of the thermogravimetry, differential scanning calorimetry, and mass spectrometry curves up to its melting temperature range (~ 1300 °C). The chemical composition and phase state of the initial and melted samples were characterized using electron-probe micro-analysis, X-ray photoemission spectroscopy, X-ray powder diffraction, and Raman spectroscopy. It was demonstrated that continuous heating (10 °C/min) up to ~ 500 °C resulting in a mass loss of ~ 5 wt. % was due to crystallization water release and dehydroxylation, while oxygen release and carbonate inclusion decomposition were observed at a higher temperature. The endothermic peak with a heat effect of 82 J/g observed at 970 ÷ 1050 °C was attributed to the charoite-to-wollastonite transition detected by real-time X-ray powder diffraction in this temperature range. Above 1100 °C, another extended endothermic effect was fixed, which was presumably due to the formation of pseudowollastonite and pre-melting processes. The melting of the charoite sample using the floating zone technique resulted in its transformation to pseudowollastonite and caused a significant color change from lilac to rose pink.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Charoite is a delightful mineral, the so-called “lilac miracle of Siberia” (Rogova et al. 2013). This stone is a rare gemstone that is well-known in the gem market due to its exciting aesthetic properties, such as unique lilac to violet color, structural features, and mechanical strength. The main (Russian Federation, Murun massif) deposit “Sirenevyi Kamen” includes both charoite and charoite-containing rocks, with a idealized formula of (K, Sr, Ba, Mn)15–16(Ca, Na)32[(Si70(O, OH)180)](OH, F)4·nH2O (Rozhdestvenskaya et al. 2010), and a complex structure of alkaline calcium silicate with tubular Si–O-radicals ((Ca1.57Na0.51K0.93Ba0.07)3.11∙Si4O10(OH0.58F0.20)0.78∙0.72H2O), Rogova et al. 2013). The chemical formula of charoite varies among different outcrops, with inclusions of aegirine, tinaksite, microcline, tokkoite and some other minerals of the metasomatic rocks also exhibits a complex association (Reguir 2001). The main charoite element components are K, Na, Ca, Si, and O, F, H2O, OH, the secondary components are Ba, Sr, and Mn; the presence of traceable amounts of Fe, Mg, Al, Ti, Zr, and Th is discovered in Murun charoite.
The nature of charoitites-forming processes has been a subject of scientific discussion. Two major hypotheses have been proposed: a magmatic origin for the charoite-bearing rocks, the charoite rocks crystallised from a fluid-saturated melt. However, the majority of researchers interpret charoitites as a typical example of metasomatic rocks (Reguir 2001).
To understand the genesis and properties of charoite it is necessary to study the behavior of the mineral upon its heating to elevated temperatures up to the melting temperature. Moreover, the undoubted interest is to investigate the possibility of charoite crystallization during the melting process. Our study is focused on the high-temperature charoite behavior and the charoite transformation upon melting during crystallization.
Experimental methods and sample characterization
Our experiments were conducted using high-quality samples of charoite. To prepare samples for analysis and melting, samples 4 × 4×40 mm and 4 × 4×1 mm were cut from a mineral block of high jewelry quality, dark lilac-violet color, without visible white, black and yellow inclusions. The content of elements in charoite was determined at IPTM RAS by inductively coupled plasma atomic emission spectrometry (iCAP-6500, Thermo Scientific, USA) and mass spectral methods of analysis (X Series 2, Thermo Scientific, USA). The elemental composition of the samples was characterized before and after thermal treatment by scanning electron microscopy (SEM, Supra 50 VP with EDX detector, Carl Zeiss).
The Raman spectra were recorded with a Princeton Instruments HRS 500 spectrometer equipped with a liquid nitrogen-cooled charge-coupled device detector. The spectra were monitored with 1200 grooves/mm grating. The samples were irradiated at room temperature with a 532-nm KLM-532/SLN-100 DPSS (FTI Optronic, St. Petersburg, Russia) laser with a laser power at the sample of about 5 mW in a back-scattering geometry. A 20 × Plan Apo Mitutoyo objective was used to focus the laser beam to a spot of approximately 3 μm in size and collect the scattered light. A razor edge Semrock beam splitter and a Holographic Tydex Notch-6 filter (Tydex, St. Petersburg, Russia) were used for laser line discrimination and acquiring the Raman spectra higher than 200 cm−1. The spectral resolution in the spectral range under study was ≈ 1 cm−1. The spectrometer was calibrated using Ne spectral lines with an uncertainty of ± 1 cm−1.
The X-ray powder diffraction data (XRD) were taken on Rigaku SmartLab SE, Cu-Kα radiation, equipped with 1D D/teX PSD in Bragg–Brentano geometry (Cu-Kα1,2, λ = 1.5418 Å) at room temperature. The data were collected in a 2θ range of 10–90° with a step of 0.01°.
The electron structure and the composition of charoite were studied experimentally by X-ray photoemission spectroscopy (XPS). The high-resolution spectra were measured using a Kratos AXIS Ultra DLD spectrometer with a photon energy of 1486.69 eV, AlKα mono, and the total resolution Ag3d5/2 was about 0.48 eV. The spectra were calibrated using Ag3d5/2 and C1s lines. The surfaces of the prepared samples were additionally cleaned by argon bombardment in ultra-high vacuum (UHV) in situ. The base pressure during the measurements was in a range of 5∙10–10–2∙10–9 Torr.
Charoite transformations at high temperatures were studied experimentally upon heating the samples from room temperature to 1300 °C in the air and argon atmosphere. NETZSCH STA 409 PC/PG was used for simultaneous thermogravimetric and calorimetric analyses from room temperature to a high-temperature of 1300 °C and allowed us to analyze the change in mass, to study the decomposition processes, thermal stability, and behavior during crystallization, and to determine enthalpy transitions, phase transition temperatures. The experiments were performed at a rate of 10.0 °C/min in an alumina crucible in argon.
Charoite rod were remelted by vertical non-crucible floating zone (FZ) melting with optical radiation heating (Balbashov and Egorov 1981). The charoite rods were FZ melted in the air, the growth rate was 10–12 mm/h at the beginning, and then 4 mm/h.
Results
Charoite composition
The average chemical composition of the initial sample was SiO2 = 52.29%, CaO = 31.42%, K2O = 7.60%, Na2O = 4.23%, BaO = 2.50% (main components, total 98.04%, Supra 50, EDX detector). Element composition is summarized in Table 1.
Charoite spectroscopy
The XRD powder pattern and Raman spectrum of the initial charoite sample are presented in Fig. 1 and agree well with the experimental data available in the literature (Buzatu and Buzgar 2010; Rozhdestvenskaya et al. 2010; RRUFF R060376).
The initial charoite sample. a The blue curve is X-ray diffraction pattern (CuKα1 radiation), the black vertical bars represent reflection positions and relative intensities reported in (Rozhdestvenskaya et al. 2010). b The representative Raman spectrum of charoite (black, present work), the Raman spectrum of charoite (red, RRUFF Project database R060376). The inset shows the high-frequency range of the spectra. The blue vertical bars represent the peak positions of the charoite reported in (Buzatu and Buzgar 2010)
Only a few studies were made on charoite (Rogova et al. 1978; Rozhdestvenskaya et al. 2009). The Raman spectra for charoite from the RRUFF Project database (Ref. (Downs 2006), R060376) are similar to the spectrum obtained in the present study. No discussions or detailed vibration mode assignments for this mineral were found in the literature. The Raman spectrum of charoite (Fig. 1, right panel) is characterized by strong fluorescence and background, the peaks are very weak, and only a few of them can be resolved. The peak positions are very close to those reported in (Buzatu and Buzgar 2010). An intensive and broad band around 2400 cm−1 is most probably of luminescence origin. It corresponds to the wavelength of ~ 610 nm, i.e., to the red region of the visible light spectrum.
Typical high-resolution XPS spectra are shown in Fig. 2. The surfaces of the prepared samples were additionally cleaned by argon bombardment in ultra-high vacuum (UHV) in situ.
The elemental composition of the charoite sample was determined by the X-ray photoelectron spectroscopy (XPS) studies after argon bombardment cleaning with core level lines of the main elements Si2p, Ca2p, Na1s, K2p, Ba3d, Sr3d, O1s, and a weak peak of F1s (Fig. 2b). The photoelectron core level spectra of oxygen O1s and silicon Si2p with binding energies of 530 eV, 532 eV and 104 eV, respectively, were characterized by a typical structure and chemical shift for silicate systems with OH and H2O.
Thermal treatment
The charoite transformation at high temperatures was studied by heating the samples from RT to 1300 °C in argon and the air. First, a lilac-violet charoite sample placed in the alumina or platinum crucibles was heated in the air in a furnace. After heating above 900 °C in the air, the violet color of charoite began to fade, and the sample melted above 1000 °C. These findings probably indicate that the structural transformations of charoite began above 900 °C and agree with the results of (Nikolskaya et al. 1976), where the charoite sample was discolored completely by annealing under the reducing conditions for three hours at 700 °C.
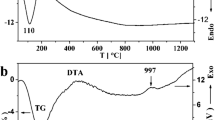
As demonstrated by the mass-spectral curves presented in Fig. 3 (right panel), upon heating, the water release (m/e = 17, 18) from the charoite sample occurred in a temperature range of ~ 50–600 °C, while the release of carbon dioxide (m/e = 44) proceeded at 500–800 °C, and that of carbon dioxide and atomic oxygen occurred above ~ 1000 °C.
a DSC (blue) and TGA (red) curves along with the mass spectrometric curve (green H2O and magenta CO2) for the initial deep violet charoite sample. The light blue and orange curves represent the first derivatives of the DSC and TGA curves, respectively, in arbitrary units. b Mass spectrometric curves: the black curve corresponds to the right ordinate axis, while the others correspond to the left one
The thermogravimetric curve measured upon heating charoite shown in Fig. 3 (left panel) demonstrates singularities at 208, 295, and 460 °C. These values are close to those of our mass spectrometry curves for water (m/e = 18) and hydroxyl group (m/e = 17), as shown in Fig. 3 (right panel), and those reported in (Matesanz et al. 2008) for the TGA and DTA dependencies. The DSC curve measured in the present work demonstrates a pronounced endothermic shift right above 200 °C and up to ~ 800 °C. It is featureless in this temperature range probably due to the overlapping of the dehydration, dehydroxylation, and carbonate decomposition processes. A deflection of the first derivative curve of the DSC signal between 500 and 560 °C might point to the peak position of the endothermic processes within this temperature range. The baseline of the DSC signal is more or less leveled above 800 °C, and above 900 °C two endothermic peaks are observed. The former narrow endothermic peak with the onset at ~ 990 °C and an enthalpy change of 82 J/g should be due to the charoite-to-wollastonite phase transition since the characteristic temperatures determined by the DSC method in the present work coincide with the temperatures obtained by the real-time XRD method and reported in (Janeczek 1991). We assume that the latter broad heat effect fixed above 1100 °C corresponds to the formation of pseudowollastonite from wollastonite and, probably, its premelting. According to the XRD phase analysis of the charoite samples subjected to isochronic annealing (Marchuk et al. 2016), the first traces of the pseudowollastonite phase appeared at ~ 1050 °C and a single phase state was attained at ~ 1250 °C. On the other hand, the synthetic wollastonite CaSiO3 transforms to pseudowollastonite at 1180 °C (Allen et al. 1906). The melting point of the latter phase is 1512 °C (Allen et al. 1906) or 1547 °C (Richet et al. 1998). The endothermic process is obviously not finished at 1300 °C (see Fig. 3); thus, a calorimeter with a higher temperature limit should be used to get a reliable DSC curve.
Hence, our direct mass-spectral and DSC/TGA experimental data confirm the assumptions on the dehydration and dehydroxylation of charoite upon heating. These assumptions were based on the results of DSC/TG and IR spectroscopy studies that were reported in (Janeczek 1991; Matesanz et al. 2008; Kaneva et al. 2020).
Floating zone melting
An attempt to grow a bulk polycrystalline sample of charoite is of undoubted interest, allowing for a reliable characterization of its physical properties and studying the high-temperature transformations of this mineral. Assuming that the growth of charoite crystals should apparently be carried out in a hydrothermal environment, in order to study the behavior of charoite under extreme thermal influences, attempts were made to grow a single crystal using non-crucible zone melting in an oxygen atmosphere. Non-crucible floating zone melting (FZM) with radiation heating is a suitable technique for growing high-quality complex oxide single crystals, as demonstrated in (Balbashov and Egorov 1981; Balbashev et al. 1996).
Charoite rod were remelted by vertical non-crucible floating zone (FZ) melting with optical radiation heating. Rods (4 × 4×40 mm) for FZ melting were prepared by cutting from the deep violet mineral block. The charoite rods were FZ melted in the air, the growth rate was 10–12 mm/h at the beginning, and then 4 mm/h. At end of melting 70 mm-long whisker was an easily retracted. As a result, the ingot in the form of cylindrical rods of 35–40 mm in length and 3–4 mm in diameter were obtained. The disk-shaped samples with a thickness of 1 mm and a diameter of 4 mm were prepared by cutting the grown cylindrical rods (see Fig. 4).
The sample color after melting and growth in the air is pale pink-lilac. According to the XRD, there are weak but rather amorphous reflections on the melted part, and the whisker is amorphous. The XRD powder pattern and Raman spectra from central part of ingot of the melted charoite minerals are presented in Fig. 5.
The charoite sample after FZ melting. a The XRD pattern (violet curve)—initial charoite, blue curve—FZ melted, and the red bars denote positions and intensities for the synthetic pseudowollastonite CaSiO3 (Yang and Prewitt 1999). b The Raman spectra of the initial charoite sample (violet) and of the charoite sample after FZ melting (black), of the synthetic CaSiO3 pseudowollastonite (red, RRUFF R080145) with the vertical bars denoting the peak positions of the synthetic CaSiO3 pseudowollastonite reported in (Richet et al. 1998) and (Chukanov and Vigasina 2020). c The high-frequency part of the Raman spectra of the initial charoite sample (violet) and one of the sample after FZ melting (black)
The average chemical composition of the melted sample in the middle part (red line area) is SiO2 = 62.79%, CaO = 24.16%, K2O = 4.28%, Na2O = 8.76% (main components, total 99.99%). Elemental composition is summarized in Table 2.
Our XRD and Raman data (see Fig. 5) demonstrate the transformation of charoite to high-temperature modification called pseudowollastonite Ca(Na, K, Ba)SiO3 having a pale pink color upon melting in the air (see also Table 2). An attempt to grow a single crystal of charoite by non-crucible floating zone (FZ) melting in an oxidizing atmosphere was unsuccessful due to the transformation of charoite into pseudowollastonite and its partial amorphization.
By using the tentative mode assignment made for wollastonite by Buzatu and Buzgar (Buzatu and Buzgar 2010) and for pseudowollastonite by Richet et al. (Richet et al. 1998), the following three frequency ranges might be distinguished in our Raman spectrum of the initial charoite sample (Fig. 1, right) and the pseudowollastonite phase (Fig. 5, right) obtained by the FZ melting procedure. The first one below 500 cm−1 corresponds to the metal–oxygen stretching modes, the second one, 500–850 cm−1, refers to the O–Si–O bending and Si-Obr stretching vibrations, and the third one, 900–1180 cm−1, corresponds to the SiOnbr stretching vibrations. However, in the high-frequency region, above 2300 cm−1 for charoite and above 1500 cm−1 for pseudowollastonite, a series of peaks is observed with a rather intensive broad band in a range of 2500–3000 cm−1. The latter band is not an overtone of the low-frequency modes; thus, we assume it to be due to luminescence with a wavelength of ~ 610 nm, i.e., in the red region of the visible light spectrum.
Conclusions
By measuring simultaneously the weight loss (TGA), heat release (DSC), and mass spectrometry (MS) curves upon heating the charoite sample to 1300 °C, we found that the dehydration and dehydroxylation processes (water and hydroxyl group release, m/e = 17, 18) occurred at 50–460 °C. Carbon dioxide release (m/e = 44) observed in a temperature range of 500–800 °C was probably due to decomposition of the carbonate inclusion in the mineral, and the heated sample lost oxygen (m/e = 16) above 1000 °C. The endothermic peak monitored at 970–1055 °C with an integral enthalpy change of 82 J/g was due to the formation of wollastonite from charoite. The melting of charoite by the floating zone procedure resulted in its transition to a pseudowollastonite-like phase.
Data availability
All data is avaliable upon request.
References
Allen ET, White WR, Wright FE (1906) On wollastonite and pseudo-wollastonite, polymorphic forms of calcium metasilicate, with optical study by F. E. Wright. Am J Sci S4-21:89–108. https://doi.org/10.2475/ajs.s4-21.122.89
Balbashov AM, Egorov SK (1981) Apparatus for growth of single crystals of oxide compounds by floating zone melting with radiation heating. J Cryst Growth 52:498–504. https://doi.org/10.1016/0022-0248(81)90328-6
Balbashev AM, Shulyatev DA, Panova GKh et al (1996) The floating zone growth and superconductive properties of La1.85Sr0.15CuO4 and Nd1.85Ce0.15CuO4 single crystals. Physica C 256:371–377. https://doi.org/10.1016/0921-4534(95)00647-8
Buzatu A, Buzgar N (2010) The Raman study of single-chain silicates. Analele Stiintifice ale Universitatii “Al I Cuza” din Iasi Seria Geologie LVI:107–125
Chukanov NV, Vigasina MF (2020) Vibrational (Infrared and Raman) spectra of minerals and related compounds. Springer International Publishing, Cham
Downs RT (2006) The RRUFF Project: an integrated study of the chemistry, crystallography, Raman and infrared spectroscopy of minerals. In: RRUFFTM Project. https://rruff.info/. Accessed 18 Aug 2023
Janeczek J (1991) Thermal decomposition of charoite. Mineral Pol 22:21–27
Kaneva EV, Radomskaya TA, Shendrik RYu et al (2020) FTIR, XRF and powder XRD experimental study of charoite: crystal chemical features of two associated generations. In: Votyakov S, Kiseleva D, Grokhovsky V, Shchapova Y (eds) Minerals: structure, properties, methods of investigation. Springer International Publishing, Cham, pp 97–104
Marchuk MV, Medvedev VYA, Ivanova LA et al (2016) Charoite experimental studies. Geodin Tektonofiz 7:105–118. https://doi.org/10.5800/GT-2016-7-1-0199
Matesanz E, Garcia-Guinea J, Crespo-Feo E et al (2008) The high-temperature behavior of charoite. Can Mineral 46:1207–1213. https://doi.org/10.3749/canmin.46.5.1207
Nikolskaya L, Novozhilov A, Samoilovich M (1976) On the nature of colours of the new alkaline calcium silicate from the Eastern Transbaikalia. Izv AN SSSR, pp 116–120
Reguir E. (2001) Aspects of the mineralogy of the Murun alkaline complex, Yakutia, Russia. MSc dissertation, Lakehead University, Ontario, Canada, 2001
Richet P, Mysen BO, Ingrin J (1998) High-temperature X-ray diffraction and Raman spectroscopy of diopside and pseudowollastonite. Phys Chem Miner 25:401–414. https://doi.org/10.1007/s002690050130
Rogova V, Rogov Y, Drits V, Kuznetsova N (1978) Charoite—a new mineral, and a new jewelery stone. Zap Vses Mineral O-Va 107:94–100
Rogova V, Rozhdestvenskaya I, Vorobjov E, et al (2013) Charoite. The Lilac Miracle of Siberia. Illustrated popular science book, 2nd edn. Petrografika, Irkutsk
Rozhdestvenskaya IV, Kogure T, Abe E, Drits VA (2009) A structural model for charoite. Mineral Mag 73:883–890. https://doi.org/10.1180/minmag.2009.073.2.883
Rozhdestvenskaya I, Mugnaioli E, Czank M et al (2010) The structure of charoite, (K, Sr, Ba, Mn)15–16(Ca, Na)32[(Si70(O, OH)180)] (OH, F)4.0.nH2O, solved by conventional and automated electron diffraction. Mineral Mag 74:159–177. https://doi.org/10.1180/minmag.2010.074.1.159
Yang H, Prewitt CT (1999) Crystal structure and compressibility of a two-layer polytype of pseudowollastonite (CaSiO3). Am Miner 84:1902–1905. https://doi.org/10.2138/am-1999-11-1217
Acknowledgements
The authors would like to thank the Shared Facilities Center at the Osipyan Institute of Solid State Physics RAS (Chernogolovka) for the use of the micro-Raman optical system and Rigaku SmartLab SE diffractometer. This work was carried out within the framework of the Governmental Program of Osipyan Institute of Solid State Physics RAS.
Author information
Authors and Affiliations
Contributions
A. I. carried out TGA, DSC and MS experiments, characterized chemical composition and phase state of the samples by means of electron-probe micro-analysis, X-ray photoemission spectroscopy, X-ray powder diffraction and prepared Figs. 1–3 and 5. D.S. conducted FZ melting and prepared Fig. 4. K. G. and O. B. carried out Raman spectroscopy experiment and prepared Figs. 1 and 5. All authors contributed to the writing of the manuscript and reviewed it.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known conflict of financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ionov, A.M., Barkalov, O.I., Shulyatev, D.A. et al. Experimental studies of charoite mineral transformations under thermal treatment. Phys Chem Minerals 51, 18 (2024). https://doi.org/10.1007/s00269-024-01282-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-024-01282-9