Abstract
Goethite (α-FeOOH) is an iron-oxyhydroxide mineral that is commonly found in soils and is of importance within the context of industrial mineralogy and aqueous geochemistry. The structure of goethite is such that vacant rows of octahedral sites form “channels” or nanopores. This study aims to investigate the response of goethite to dynamic shock compression in order to advance our understanding of minerals as potential shock-absorbing media. Shock compression of synthetic goethite powdered samples was achieved by using an inverted shock microscope and laser driven “flyer plates”. With this setup, a high-energy laser launches small aluminum discs as projectiles or flyer plates at velocities of the order of a few km/s towards the sample. The resulting impact sends a shock wave through the sample, thereby compressing it. The compression is precisely controlled by the plate-impact speed, which in turn is controlled by laser-power. In this work, 25 µm aluminum flyer plates with 3.5 km/s impact velocities were used. The impact resulted in a planar shock wave with shock velocity (Us) ~ 6.78 km/s and an estimated pressure of ~ 41.6 GPa. The shock wave compressed the target goethite for 5 ns. Subsequent, post-shock investigations via transmission electron microscopy (TEM) documented that crystal morphology persisted, and that goethite’s “bird’s nest” texture was maintained. Lattice fringe images revealed localized zones of distortion and amorphous regions within single goethite particles. Raman spectra appear to indicate structural changes after shock compression with the shocked goethite spectra matching that of synthetic hematite. X-ray diffraction (XRD) interestingly identified two major phases: goethite and magnetite. Irrespective of the mineral phases present, the goethite particles persist post shock. A thixotropic-like model for accompanying shock compression is proposed to account for goethite’s shock resistant behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The physicochemical properties of iron oxide and oxyhydroxide minerals have been widely studied due to the variety of their applications within the field of industrial mineralogy and their associated economic value (Cornell and Schwertmann 2003). These minerals are naturally found in soils and regolith on Earth, in addition to being present on other planetary surfaces (e.g., Christensen et al. 2000; Rampe et al. 2020), and are among the most common, chemically simple minerals in the environment. Specifically, they are known to dominate heavily weathered soil horizons and often develop as a result of weathering and oxidation of more complex iron-bearing minerals (Schwertmann et al. 1989; Ruan et al. 2001; Cornell and Schwertmann 2003; Gleason et al. 2008). Examples of their use range widely from catalysis in reactions to production of electrochemical batteries (Cornell and Schwertmann 2003; Ramimoghadam et al. 2014). Hence, a comprehensive understanding of properties and behavior in various pressure–temperature conditions of oxide and oxy-hydroxide minerals is vital for supporting emerging technology developments and improvements on existing methods and products.
Goethite (α-FeOOH) is one of the most stable iron-oxyhydroxide minerals in the near-Earth surface environment (Schwertmann et al. 1989; Cornell and Schwertmann 2003). It plays important roles in soil science and aqueous geochemistry owing to its high surface adsorption and its role as a scavenger for heavy metals (e.g., Kosmulski and Mączka 2004). As a result, goethite is often used as an eco-friendly material alternative in environmental remediation projects which aim to reduce the presence of heavy metals such as cadmium and nickel (e.g., Cornell and Schwertmann 2003; Ma et al. 2015, Frierdich et al. 2019; Dash et al. 2020). Historically, goethite has also been used in the production of brown and yellow pigment dating back to 23,500 years ago in cave drawings (Schwertmann and Cornell 1991; Pomiés et al. 1998; Cornell and Schwertmann 2003; Pomiés et al. 2007).
Goethite is a member of the orthorhombic space group Pbnm with unit cell parameters of a = 4.587 Å, b = 9.937 Å, and c = 3.015 Å and a unit cell volume of 138.37 Å3 (Bernal et al. 1958; De Faria et al. 1963; Gualtieri et al. 1999; Cornell and Schwertmann 2003). This space group is also commonly written as Pnma which defines goethite as having the same symmetry elements but with its crystallographic axes defined differently. In the Pnma notation, unit cell parameters are a = 9.95 Å, b = 3.01 Å, and c = 4.62 Å (Szytuta et al. 1968; Gualtieri et al. 1999; Cornell and Schwertmann 2003). This notation is often used when making crystallographic comparisons with hematite such that the c axis of hematite is then 3 times the length of goethite. (Gualtieri et al. 1999). The structure of goethite is heavily dictated by its hexagonal close packing (hcp) structure of oxygen and hydroxide atoms where ferric iron (Fe3+) ions fill half the octahedral sites creating double chains of edge sharing octahedra as illustrated in Fig. 1. Positioning of these low density vacant octahedral sites extend throughout the length of the crystal forming connected “channels” (Waychunas et al. 1991; Ruan et al. 2001; Cornell and Schwertmann 2003). These channels are hypothesized to play a role in mechanisms related to compression attenuation and help facilitate diffusion of adsorbed contaminants (Nagai et al. 2003; Fan et al. 2006; Bassel et al. 2020).
In addition to applications within the context of environmental remediation, goethite is a critical industrial mineral due to its close structural relationship to hematite (e.g., Cornell and Schwertmann 2003; Fan et al. 2006). The transformation of goethite into hematite occurs at relatively low temperatures (250–800 °C) and is strongly dependent on particle size, shape, and pressure conditions (e.g., Cudennec and Lecerf 2005; Fan et al. 2006; Gianella et al. 2010). The rate at which goethite particles lose their hydroxyl groups is directly proportional to their surface area (Bernal et al. 1958; Fan et al. 2006; Gianella et al. 2010) with pressure acting to stabilize the goethite structure (Voigt and Will 1981; Gleason et al. 2008). The transition of goethite to hematite has been extensively investigated to evaluate the possibility of a simple topotactic transition or if the transition is associated with the emergence of intermediate phases such as protohematite or hydrohematite. (Bernal et al. 1958; Wolska et al. 1981, 1988; Brugina et al. 2000; Cudennec et al. 2005; Fan et al. 2006; Wells et al. 2006; Zhang et al. 2010; Mote e Nogueira et al. 2022). Understanding this transition under a variety of different physical conditions is therefore important for identifying the specific mechanisms responsible.
Previous studies which evaluated the structural integrity of goethite investigated samples under static compression using diamond anvil cells (DAC; Williams et al. 1996; Nagai et al. 2003; Gleason et al. 2008; Liu et al. 2019; Tang et al. 2020). These studies found that under static high-pressure conditions the lengths of crystallographic axes decreased with increasing pressure and were accompanied by no major structural changes up to 24.5 GPa. (Nagai et al. 2003; Gleason et al. 2008). These DAC experiments established ultrahigh pressure (5–200 GPa) conditions along the isotherm of the given sample (Nagai et al. 2003; Tang et al. 2020). Alternatively, compression can also be achieved using the propagation of a shock wave front to create high pressure pulses (20–60 GPa; Dlott 2011). While the effects of dynamic compression have been investigated in other common rock-forming minerals, often to explore shock metamorphism, reports on specific effects on goethite remain scarce and consist of several open questions (Wackerle et al. 1962; Short et al. 1966; Chao et al. 1967). Specifically, previous studies focused on quartz and plagioclase and revealed that shock metamorphism can result in phase transformations (e.g., quartz → coesite), fusion, and the development of microstructures such as planar deformation features (PDFs; Wackerle et al. 1962; Chao et al. 1967; Offield et al. 1987; Stoffler and Langenhorst 1994). It is noteworthy that there are open questions regarding the shock induced phase transition in goethite. While some previous studies (Gleason et al. 2008; Liu et al. 2019) have found α–ε phase transitions between 7 and 20 GPa pressures, others (for example, Tang et al. 2020) have not seen any such effect. Direct and statistically consistent experimental evidence on goethite could provide critical information on the mechanisms associated with shock metamorphosis.
Dynamic compression experiments have recently been used to investigate chemical kinetics, structural changes, and mechanochemistry in materials (Bhowmick et al. 2018; Zhou et al. 2019; Bassett et al. 2020; Nissen et al. 2021a, b; Bhowmick et al. 2023). With recent laser-based table-top plate impact techniques, shock compression investigations have become popular in high-pressure studies of condensed matter due to its precisely controllable pressures through tunable laser pulses, economic feasibility, low uncertainties, and high repeatability features (Bhowmick et al. 2018). Because of the high-throughput and reliability, this technique is also valuable as feedback for theoretical modeling. Laser driven flyer plate impact experiments have been able to help theoretical models where parameters from compression experiments led to better simulation (Stekovic et al. 2021).
The purpose of this study is to investigate the effects of dynamic compression on the structure and stability of synthetic nanocrystalline goethite. The applied pressure of 41.6 GPa is well above the reported phase transition thresholds that could occur between 7 and 20 GPa (Liu et al. 2019) but slightly below the pressure that could trigger symmetry change effects at ~ 43 GPa, or the second order transition at 90 GPa to form pyrite-FeOOH (Tang et al. 2020). For applications related to shock assisted synthesis pathways, or applications related to shock energy absorption, it is important to investigate and characterize the physicochemical effects of dynamic compression through spectroscopic studies. In this work, a relatively new experimental setup of laser-driven flyer plates is used to subject goethite to high pressure. Goethite samples recovered after the shock compression experiments were characterized using transmission electron microscopy (TEM), confocal Raman spectroscopy, and powder X-ray diffraction (XRD) to identify any structural and/or phase transformations.
Materials and methods
Materials
In this work, synthetic crystals of goethite were used instead of naturally occurring goethite in order to eliminate observations associated with cation impurities that could be present in natural samples. As discussed earlier, goethite acts as a scavenger mineral and in the environment will adsorb common transition metals (e.g., Cd, Cu) onto its structure (Waychanus et al. 1991; Gialanella et al. 2010). Incorporation of such metals has the potential to influence the effects of the shock compression on its structure.
Goethite used in this experiment was synthesized using the method described in Schwertmann and Cornell (1991). In this approach, 180 ml of 5 M KOH is added to 100 ml of 1 M Fe(NO3)3•9H2O solution while being stirred. Using double distilled water, the mixture was diluted to 2 L and stored at 70 °C for 60 h. Precipitates of this solution were then collected using a centrifuge for 15 min at 3000 g (~ 10,000 rpm). Precipitates were rinsed with double distilled water 3 separate times for 3 h each and subsequently dried in an oven at 40 °C. Once dried, goethite was finely ground into a powder and stored at room temperature.
Shock compression experiments
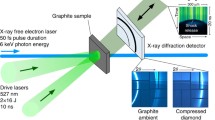
The system implemented to perform shock compression experiments was developed and characterized by the Dlott research group at the University of Illinois Urbana-Champaign (Dlott 2011; Bhowmick et al. 2018; Bassett et al. 2020). This system uses an inverted shock microscope with laser driven projectiles (flyer plates) to send a shock wave through the target material (see Figs. 2, 3). Reproducibility and precision of the laser-driven flyer plates, as well as its applications as a scientific tool for novel dynamic compression experiments, has been established and previously documented (Bhowmick et al. 2018; Zhou et al. 2019; Bassett et al. 2020, Nissen et al. 2021a, b). Specifically, the shock compression apparatus uses a high energy laser to launch small (0.5 mm in diameter) metal discs at high velocities before impacting the sample and compressing it. The pressure applied to the sample is directly proportional to the impact velocity of the flyer plate. Each plate-impact experiment is a single-shot, irreversible event where the sample is taken from ambient (unshocked) state to a certain pressured state. Each single-shot experiment thus connects an unshocked sample to its shocked (pressurized) state on the Hugoniot equation of state. Parameters such as shock velocity (Us), particle velocity (Up), density (ρ) and pressure (P) in any media are obtained from the shock Hugoniot equations through impedance matching calculations (Forbes 2012). In many cases, the equation connecting Us and Up represents a straight line given in the form:
where A is the speed of sound in the material, and B is the slope of the straight line. The shock Hugoniot equation for goethite at high-pressure was reported recently in Gan et al. (2023) as:
Summary schematic showing the table-top shock compression microscope setup. The sample holder and flyer plate operation are located inside the target chamber (see Fig. 3)
The stress in a material impacted by a flat plate can be determined using the impedance matching technique, which gives the following equation (Forbes 2012):
where ρ0 is the density of the unshocked bulk mineral material, which is typically ~ 4.28 g/cm3 in goethite. However, the initial densities reported recently (Gan et al. 2023) for FeOOH varied significantly from the typical value and was measured to vary between 3.901 and 3.920 g/cm3. In this work we are using an average of the two, which is 3.910 g/cm3. All shock compression experiments in this report have been performed with 25 µm flyers, launched with an impact speed of 3.5 km/s. Using flyer velocity of 3.5 km/s, Al and FeOOH Hugoniots, and impedance matching for the Al-FeOOH interface, a particle velocity (Up) of 1.57 km/s was found. The shock velocity in goethite was determined to be 6.78 km/s. and the pressure was estimated to be ~ 41.6GPa. The density of shocked goethite (ρ) was calculated using the following equation (Forbes 2012):
Equation (4) yielded a calculated density of 5.09 g/cm3. Uncertainty from the presence of variable amounts of nanoporosity and the variability of texture in goethite powders do not permit precise density values to be determined. However, the values determined above are reasonably close to the previous reports where FeOOH Hugoniots were discussed (Gan et al. 2023; Zhou et al. 2023). Future studies involving precise density values would require computational modeling owing to these factors and the adsorption of atmospheric water under the experimental conditions, which is outside the scope of this work.
Figures 2 and 3 provide an overview of the tabletop shock compression setup. In this experiment a Nd:YAG laser (Spectra-Physics Quanta-Ray Pro-350-10) of wavelength 1064 nm, with a maximum pulse energy of 2.5 J, a pulse width of 20 ns, and a high value of M2 = 40 is used to launch flyer plates. Flyer plates in the experiment are generated from thin metal foils epoxied to glass plates which measure 7.62 cm × 7.62 cm and are 6.35 mm thick. Each flyer plate is a disc approximately 0.5 mm in diameter, moving with its flat surface parallel to the sample plane. While several different kinds of metal foils can be used (such as Al, Cu, or stainless steel), 25 µm thick Al foils have been used in this work. Selection of metal foil is dependent on characteristics of the sample such as reactivity, initiation threshold, and optical parameters. Aluminum has been selected in most flyer plate experiments because of the availability of Al foils of various thicknesses, a well-developed Hugoniot for the material, and its lower susceptibility to chemical changes (such as oxidation). Flyer velocities are precisely controlled through the power of the laser and the shock duration is controlled through the thickness of the foil used. The setup is versatile and able to accommodate both solids and liquids (Dlott 2011). For materials transparent to 1550 nm optical signals, flyer velocities and pressures are determined by a photon Doppler velocimeter (PDV) linked to the system. In cases such as these where goethite is opaque to the optical signal, impedance matching calculations are used (as described above).
All experiments were performed using 25 μm Al flyer discs with 3.5 km/s impact velocities. Each plate impact experiment consistently produced a single shock wave that propagated for 5 ns duration through the sample. Impact velocity and flyer plate thickness are consistent with previous experiments performed on cryptomelane (Murchland et al. 2024). Reproducibility of flyer velocities (0.56%) and impact duration (± 0.81 ns) ensured that each single shot experiment was performed under the same conditions. The sample, or “target” in these experiments refers to a polyimide well, filled with powdered goethite. The targets were prepared by pouring the powdered sample into small wells. The wells (Ф = 2 mm) were made by polyimide tapes of 90 μm thickness. The powdered crystals were gently pushed into the wells using a spatula and were visually inspected through an inverted microscope which is an integral part of the shock apparatus. Using the microscope, before launching an impactor to the sample wells, it was made sure that the respective well looked normal, and there were enough samples in it. Only the samples that were reasonably flat as observed through the microscope were shocked. However, due to the porosity and uneven nature of the samples, it was not possible to quantitatively characterize the flatness of the samples.
As each shot destroys the sample this poses a challenge for high-throughput spectroscopy under shock hence a versatile and inexpensive sample array was used (Bhowmick et al. 2018; Nissen et al. 2021a, b). The sample array consisted of a polyimide or Teflon adhesive tape on a glass substrate with 77 laser-milled microcuvettes spaced ~ 0.5 mm from each other. Given the proximity of each well to one another, the potential exists for nearby wells to be impacted by the same shot. This was avoided by visually inspecting the samples in the inverted microscope before initiating the launch.
Transmission electron microscopy
Transmission electron microscopy (TEM) was used to initially characterize both unshocked and shocked material. For unshocked material, samples were prepared by suspending a small amount of nanocrystalline material in ~ 2 ml of ethanol in a glass vial, capped, shaken, and allowed to settle for 30 s. The suspended solution was then carefully dropped onto a 3-mm copper grid with lacey-carbon film and allowed to air-dry. Preparation for the shocked materials was performed by retrieving material from the center of the microcuvettes. Rather than creating a large suspended solution and leaving it to settle, a mixture of ethanol and shocked material was gathered into a suspension bead on a glass slide and quickly drawn into a pipette before carefully being dropped onto a 3-mm copper grid with lacey-carbon film and allowed to air dry. Analysis of both pre- and post-shock compression were performed on a JEOL JEM-2100 transmission electron microscope (TEM) operating at 200 kV. This system images up to 1.5 millionX allowing for structural imaging of crystal lattices and is equipped with a Bruker EDS detector that was used for chemical analysis and the identification of any potential contaminants in the synthetic material. Images were acquired using a Gatan Orius SC 200D CCD camera. Selected area electron diffraction patterns (SAED) were used to support crystallinity interpretations. This approach of postmortem analysis has been used and cited for the characterization of other minerals such as cryptomelane (Cymes et al. 2020, 2021; Murchland et al. 2024). The following challenges associated with this approach are noted here as being the small amount of sample material and whether enough material has been analyzed such that changes can be observed.
Confocal Raman spectroscopy
To investigate any changes to the vibrational energy mode of goethite after dynamic shock compression, Raman spectroscopy was performed. Powdered samples were obtained pre- and post-shock. Analyses were performed at the University of Illinois Urbana-Champaign using a confocal Raman microscope (Horiba LabRam HR) with a 100 × /0.95NA objective and a 633 nm excitation laser. Each spectrum was collected directly from the microcuvettes from an area of about ~ 1 µm in diameter with an acquisition time of 10 s and the average of 3 spectra taken from the same spot which reduced background noise. While Raman spectroscopy is generally considered to be a non-destructive process, goethite will transform into hematite at relatively low temperatures. To ensure that the excitation laser would not “burn” the recovered sample, acquisition time and intensity of the beam were lowered such that all observations derived from the measurements were attributable to result of the shock compression experiment.
X-ray diffraction
Powder X- ray diffraction (XRD) was performed on unshocked and shocked material using a Bruker D8 Advance powder X-ray diffractometer at Miami University to confirm the presence of other iron oxide phases. Shocked material was collected from the center of the wells and mounted onto “zero” background holders themselves which are cut 6° off the c-axis of synthetic quartz. Data on both samples were collected from 4° 2θ to 75° 2θ, with a step size of 0.01° 2θ at 2 s per step, using Cu Kα radiation for a total 4 h run time. Accompanying Bruker software DiffracEva and the Powder Diffraction File (PDF) database were used for phase identification.
Results
Transmission electron microscopy
Unshocked, synthetic goethite grains were first characterized and imaged using TEM and found to be consistent with previous characterization studies (Watari et al. 1979; Goss et al. 1987; Gialanella et al. 2010). The morphology of studied goethite nanocrystals can be described as acicular with a range of particle sizes from ~ 0.5 to 1 µm in length and from ~ 0.1 to 0.3 µm in width (Fig. 4a, b). Clusters of individual goethite crystals were commonly observed throughout the sample which are defined here as exhibiting a “bird’s nest” texture (Fig. 4c). These range from 1 to 2.5 µm in width and from 1.5 to 3 µm in length. Selected area electron diffraction (SAED) patterns were collected on several goethite grains as illustrated in Fig. 4d–f. SAED patterns and spacings observed were again consistent with previous characterization studies of crystalline synthetic goethite (e.g., Dong et al. 2003). Structural imaging of unshocked goethite was also performed in order to document the highly crystalline natural of the material. No impurities or distortion to the lattice fringes were observed (see Fig. 5). The d-spacing measurements of the lattice fringes were found to be ~ 4.54 Å. This is consistent with previous studies (e.g., Nagai et al. 2003).
Transmission electron microscopy was then performed on shocked goethite material as a comparison. Minor changes to its texture and morphology were observed. Goethite’s “birds’ nest” texture was maintained and consistently observed throughout the shocked sample (Fig. 6a). Individual goethite grains have the same acicular shape with particle sizes ranging from ~ 0.1 to 0.5 µm in width and from ~ 0.5 to 1.5 µm in length on average (Fig. 6b, c). Although no major morphological changes were observed in the sample, amorphous and disjointed lattice fringe regions within crystals were observed (see Fig. 7). SAED patterns of the shocked material were also obtained and are consistent with the original unshocked sample. The only difference noted is more streaking and less uniformity of the reflections (Fig. 6e, f). High resolution structural imaging of shocked goethite was performed on several individual crystals and on a cluster of aggregates. Imaging indicates that the shocked goethite material displays a high level of crystallinity with well-defined lattices through much of the crystal volume (Fig. 7a, b). However, areas located near crystal faces of several particles showed evidence of lattice fringe distortion (Fig. 7a, b). Several d-spacing measurements of lattice fringes were acquired from individual shocked crystals and compared with measurements from the initial starting material. Unshocked goethite crystals had fringes that were consistently measured at ~ 4.54 Å. The d-spacing measurements conducted on individual shocked goethite crystals were consistently calculated to be ~ 4.056 Å, significantly lower than the starting ~ 4.54 Å but consistent with static DAC experiments with goethite (Nagai 2003), indicating that major structural changes have occurred. Collectively, lattice fringe distortion and changes in d-spacing, combined with the lack of uniformity of diffraction reflections and streaking, are interpreted to be the result of shock.
Bright field TEM images of shocked goethite. Upper left panel shows several examples of the “birds nest texture’ preserved with a SAED from the aggregate goethite in the center of the upper left image. The middle and right panels (and paired SAED) show shocked goethite aggregate particles retaining much of their crystallinity
TEM images shown at different scales show the nature and distribution of lattice fringes for shocked goethite. The boxed regions in A and B (left) are shown, respectively, to the right at higher magnification. Pseudo-waveform of lattice fringes in the A row, and amorphous and disjointed lattice fringe arrangements in the B row, are interpreted to be the result of shock deformation. The images above are examples of where euhedral crystal faces are preserved but where defects of a variety of types occur as a result of shock deformation
Confocal Raman spectroscopy
Raman measurements were carried out on starting goethite and post compression materials. For both materials, measurements were collected from several locations moving from the center to the edge of the crater. Given that this is a powdered sample, slight variability between each Raman measurement is expected. Raman spectra data was collected on unshocked goethite first (Fig. 8a–c). The data presented in Fig. 8a was collected at the center of the well while the data presented in Fig. 8c was collected near the rim of the well. Results of unshocked goethite document distinct peaks at 255, 309, 392, 493, and 560 cm−1 (Fig. 8a–c). These are all consistent with well-established vibrational modes for goethite (Dunnwald 1989; Kustova 1992; De Faria 1997; Legodi 2007; Tang 2020). For analysis of individual Raman peaks, assignments detailed in Legodi (2007) and Liu (2019) were used. The peaks at 255 and 392 cm−1 correspond to symmetric stretching of the Fe–O and Fe–O–Fe/OH bonds in the structure. The peaks at 493 and 460 cm−1 correspond to asymmetric stretching of Fe–OH bonds.
A, B, C Raman Spectra of unshocked goethite powdered samples. Note that since this is a powdered sample with random orientation slight variation between spectra occur. D, E, F Raman spectra of goethite after undergoing dynamic shock compression experiments taken at different distances from the impact crater. Panel D is associated with the center of the impact crater while panel E is associated with the rim of the crater
Spectral data of goethite recovered after shock compression experiments document distinct peaks at 234, 301, 420, 518, 627, 680, and 1343 cm−1 (Fig. 8d–f). Consistent with the approach for unshocked samples, spectra were taken from different locations starting at the center of the crater and moving outward to the edge of the crater. The data presented in Fig. 8e was recorded at the center of the impact crater with Fig. 8f documenting the edge of the crater. No significantly different patterns were observed at different locations. Data collected from the center of the crater (Fig. 8d) is nearly identical to the signature close to the rim (Fig. 8f). As shown in Fig. 8, shocked goethite shows an overall shift in most of the peaks with the addition of new peaks at 627 and 680 cm−1. This indicates significant structural changes which are interpreted to be a result of the shock compression experiments.
Given the phase transformation that occurs between goethite and hematite, Raman spectra was also collected on synthetic hematite in order to document any similarities between the signatures. Hematite spectra were acquired using the same approach that was used for goethite with peaks at 234, 298, 418, 503, 619, and 1343 cm−1 (Fig. 8d–f). This signature is consistent with previous spectral measurements of this material (Kustova 1992; De Faria 1997; Shim 2002; Hanesch 2007). The peaks of synthetic hematite and shocked goethite are nearly identical. Figure 9 provides a clearer view of these two patterns showing near identical peaks with the exception of a new peak at 673 cm−1. This can be attributed to Fe–O stretching vibrations. The spectra of both unshocked and shocked material show near identical peak intensities. In Fig. 9, spectra are artificially shifted upwards to offer better comparison.
Upper panel—comparison of unshocked and shocked goethite Raman spectra. Note the shifting of peaks at 255 and 392 cm−1 with the formation of new peaks at 627 and 680 cm−1. Spectra was shifted up 500 arbitrary units to offer better comparison since intensity was only minimally affected. Lower panel - comparison of shocked goethite and unshocked hematite. Spectra was shifted up 800 arbitrary units to offer a better visual comparison
X-ray diffraction
Starting unshocked goethite was confirmed to have no impurities or other iron oxide phases by matching powder diffraction data to PDF card #81-0464 (Fig. 10a). The shocked sample revealed the presence of numerous other iron oxide and iron aluminum phases (Fig. 10b). However, goethite is still demonstrably the most dominant phase present as indicated by major d-spacing peaks at d(110) = 4.18 Å, d(120) = 3.38 Å, d(111) = 2.45 Å. Strong Al peaks are also observed and are attributed to the addition of the Al flyer plates into the system. A major reflection peak is located at 2.93 Å which most closely matches the d(220) reflection peak of magnetite. Small traces of hematite and hercynite (spinel) are present as well. Two low intensity and disordered peaks occur at ~ 42 and 45.8 2θ which are not identifiable.
Discussion
Data interpretation
Given the fine-grained nature of the starting goethite material and the relatively low temperature at which goethite transforms into hematite (250–800 °C), it was unexpected that goethite would largely persist post shock as coherent particles. At low magnification TEM imaging (100,000–250,000X) of individual crystals showed diagnostic characteristics of goethite (see Fig. 6). Crystals showed minor change after dynamic shock compression experiments with the small elongated acicular habit being preserved. At higher magnification TEM imaging (1.2–1.5 millionX) of lattice fringes indicates areas of distortion located mostly near the crystal faces (see Fig. 7). Some amorphous regions were also observed via TEM near these crystal faces. Raman spectra appear to indicate structural changes after shock compression with the shocked goethite spectra matching that of synthetic hematite (see Fig. 8). The changes are consistent with previous reports where changes in line widths and peak splitting have been found (Tang et al. 2020). Powder XRD data however, shows that goethite remains the dominant phase in the shocked material with hematite present in only trace amounts and with magnetite (another iron oxide) more abundant (Fig. 10). Some factors to be considered when comparing results amongst post shock characterization are: 1) variation in pressure and temperature regimes locally in individual sample craters, which have the potential to hypothetically produce multiple phases, have not been experimentally determined; 2) the volumes of shocked samples characterized are different amongst the respective techniques. Variations in beam sizes, angle dependent features, and ratio of shocked versus unshocked materials sampled have the potential to induce sample bias. The differences between observations reported in this study and those reported by previous spectroscopic investigations could be due to the difference in pressure loading techniques (Dlott 2011), but also due to simultaneous presence of multiple phases in the samples shocked to ~ 39.41 GPa. Previous studies of high-pressure goethite have also observed variations in post experiment sample(s). For example, XRD data in DAC studies have not presented any evidence of phase transitions up to 24 GPa, while Raman studies reported a α-ε transition at only 7 GPa (Tang et al. 2020). Hence, the differences in observations from TEM, Raman, and XRD data are not entirely surprising. In spite of the observational differences, the interpretations for what is likely happening to goethite due to shock compression can mostly be explained. It is acknowledged that while goethite particles are coherent in shape, there may be multiple phases or transformations present within a given goethite particle. Detailed high resolution TEM studies of oriented particles is recommended for future work.
As indicated by TEM and XRD data, goethite particles largely persisted through the dynamic shock compression experiments. This could be an indication of some degree of shock resistance. In shocked goethite, features from the unshocked mineral are still strongly present as confirmed by powder XRD data and TEM analysis. It is possible that TEM and XRD were unable to detect the highly localized changes otherwise documented via Raman, which may involve oxidation state and local bond environment properties. Evidence of other iron oxide phases, and some disorder to the crystal structure of goethite, have been observed in XRD and TEM. In SAED patterns of shocked goethite, a lack of uniformity and streaking indicate some disorder to goethite’s crystal structure while high magnification structural imaging revealed areas of lattice distortion and amorphous regions near crystal faces. These are directly interpreted as effects of the shock compression.
Some previous work shows broadly similar behavior observed in elongated nanoparticle morphologies which are congruent with our observations of goethite. Zhu et al. (2003) investigated WS2 nanotube material at impact velocities of 1.0 and 1.6 km/s, generating 21 and 36 GPa peak pressures and reported and assessed damage after shock compression. Zhu et al. (2003) found that severe damage can be localized closer to crystal terminations but also along the entire nanoparticle where the outer layers toward the end of the tube being removed progressively with the most significant damage exhibited by partial exfoliation of tube walls. However, in Zhu et al. (2003) approximately half of the walls, including the cores of inner oxide were destroyed such that tube morphology was nearly unrecognizable. Zhu et al. (2003) also found that some of the WS2 nanotube particles withstood a pressure of 21 GPa and report that some post-shock nanotubes were functionally the same as starting material having long, straight, morphologies and open preserved tube terminations. Zhu et al. (1998) investigated shock effects on carbon nanotubes at 50 GPa and found that tubes collapse, a few outer shells separate, several dislocations develop in tube walls, and the tube inner cores become diffuse or collapse. Zhu et al. (1998) suggest that the tubular nanostructures have high mechanical strength which had been predicted theoretically and the pressure resistance of the arc-discharge-produced type of carbon nano tube is remarkably high.
In this study, from the acquired Raman data, it is strongly suggested that a transformation from goethite to hematite has (in part) occurred. This is interpreted as a result of shock transformation owing to an increase of either (or both) pressure and temperature. Goethite, hematite, and magnetite are structurally very similar to one another with a very similar oxygen framework. Hence, phase transformation between these minerals would be expected with changes of temperature and pressure. The regions of lattice fringe distortion and localized amorphous regions in goethite particles suggest shock energy is variably dissipated through goethite particles. Although the pressure applied is well above the reported α-ε phase transition threshold, any such evidence was not found in this study. While there are some changes recorded in the Raman, the XRD data did not show any such phase transition. It might be noteworthy that a similar result was found in a recent report (Tang et al. 2020), where no phase transition or formation of ε-FeOOH was evidenced in the XRD data up to a pressure of 32 GPa, while small changes were noticed in Raman spectra. This result could be understood by considering that ε-FeOOH is a high-pressure-high-temperature phase and is not quenchable after the pressure is released.
Proposed mechanism
In this work, TEM analysis of pre- and post-shock goethite has demonstrated that it maintained its particle morphology and texture after dynamic shock compression experiments with only small areas of lattice framework distortion (see Figs. 6, 7). Goethite’s “bird’s nest’ texture of nanocrystal aggregates was observed post-shock with structural imaging documenting localized distortion of the goethite crystal lattice and a shortening of the lattice fringe spacing. Henceforth, to explain goethite’s shock resistant behavior a thixotropic-like model is proposed (Fig. 11). This model was previously proposed in Murchland et al. 2024) to explain the shock resistant nature of cryptomelane, a manganese oxide mineral with a tunnel structure and relatively high nanoporosity. This mechanism proposes that pores within the structure allow the mineral to move, align, and accommodate shock shear forces before they rebound back to near its original shape and position. Goethite appears to respond similarly. Similar to the nanopores in cryptomelane, channels that run the length of the crystal may allow goethite to move and accommodate the forces induced by a shock wave. When under compression, individual crystals may then collapse in upon themselves before rebounding back post shock. Nanopores forming during the dehydroxylation of goethite have been imaged and detailed in previous studies (Goss 1987; Walter 2001; Jia 2015). These nanopores help accelerate the transition of goethite into hematite and are being proposed here as a mechanism for accommodating stress loading during shock compression.
Schematic summarizing the thixotropic-like mechanism of goethite experiencing dynamic shock compression as also presented in (Murchland et al. 2024). Mineral nanopores compress under the shock wave which attenuates deformation. Rebound occurs after the shock wave has passed (relaxation)
It is noted that Gao et al. (2023) investigated the Hugoniot equation of state in goethite and measured up to ∼ 90 GPa and ∼ 2100 K using a two-stage light-gas gun. Their experiments indicate that goethite exhibits a density discontinuity between conditions of 47 GPa (∼ 950 K) and 61 GPa (∼ 1150 K). Gao et al. (2023) attributed this density change to the high-low spin transition of Fe3+ and further indicate this transition is consistent with their first-principles calculations performed. The work of Gao et al. (2023) therefore shows that goethite is stable at pressures below 47 GPa. The experiments of the present study are associated with pressures of 41.6 GPa (see earlier) and support the findings of Gao et al. (2023) however, it is acknowledged that sample heterogeneity and some possible reactions with Al flyer were detected locally in the recovered sample.
While one mechanism alone cannot fully explain the behavior of shocked goethite, the thixotropic-like model seems like the most viable explanation under the conditions applied here. Preferential stacking and the arrangement of the hexagonally closest packed oxygen may also help explain a mechanism at play. The magnetite-hematite-goethite phase transformation series exhibits one of the most frequent crystallographic preferred orientations where basal planes in goethite correspond to basal planes in hematite which correspond to octahedral planes in magnetite. As a result, oxygen lattices are preserved in the framework of the mineral and the transformation happens topotactically (Cudennec and Lecerf 2005). This preferred orientation may therefore allow goethite to accommodate applied shock forces without major structural modifications.
Future work and implications
Results of this research have the potential to contribute to a variety of different fields and industries. As discussed earlier, goethite is an important mineral in soils and has been documented to occur on the surface of other planetary objects throughout the Solar System, most notably Mars (e.g., Huguein 1974; Klingelhöfer et al. 2004; Chen et al. 2021). In addition, high pressure studies of goethite have provided evidence of FeOOH phase stability in Earth’s mantle and could contribute to the understanding of seismic discontinuity (Zhou et al. 2023). Gaining a better understanding of goethite’s stability under shock compression is therefore potentially insightful for investigations of the formation and evolution of impact-related features and the interior structures of other differentiated planetary objects. Like the gas-gun experiments that have been used to simulate meteorite impacts, the tabletop laser-driven flyer disc setup utilized in this experiment offers a more economically viable approach without compromising precision and reliability. This research also expands on the growing use of laser driven shock apparatuses within a geologic framework.
Goethite’s shock resistant nature, along with its tunnel structure, make it an interesting material for potential insulation applications in highly turbulent environments such as blasts. Future studies could also concentrate on collecting data from oriented, macrocrystals, of goethite to better understand details of phase transformations. Combined with a postmortem confocal Raman investigation on such macrocrystals, results would help establish goethite’s potential for blast mitigation applications. For such applications, it is important to thoroughly characterize the energy landscape of shocked versus unshocked goethite (Zhou et al. 2019). Goethite is a readily available commercially and would potentially be economically feasible within these contexts. While more research is needed to evaluate goethite’s suitability, the material shows promise for a range of shock-related applications.
While this work offers an initial investigation into the stability of goethite and its shock resistant behavior, more experiments should be implemented in the future to further document and interpret its response to shock. In the experiment presented here, goethite was investigated under one impact velocity: 3.5 km/s. Lower velocities could be explored to determine the minimum velocity required to induce changes to goethite. Establishing boundary conditions for phase transformations and/or determining when goethite becomes an amorphous iron oxide, will not only provide better understanding of the mechanism at work but will also clarify potential applicability. Most areas of distortion were located near fringes associated with crystal faces hence an interesting future study would be to analyze and image crystals down a preferred crystal axis orientation. Imaging down an axis would provide further insight into how the crystal is dispersing the force induced by the shock conditions through identification of deformation and/or phase changes.
Cation doping of goethite with metals such as Al and Ti could be used to assess the role that bond strength has in the thixotropic-like model being proposed here. The bond strength of the Fe3+–O bond is only 390 kJ/mol which can easily be substituted with Al3+ to increase the strength to 512 kJ/mol or to 662 kJ/mol when substituted with Ti4+. At present, the effects that changing the bond strength of the mineral has on its shock resistant nature is an area that should be explored. Increasing the bond strength may increase the rigidity of goethite’s structure such that the thixotropic-like model of compression of these low-density regions can no longer be accommodated. Similar to changing the bond strength with other transition and rare-earth metals, polymorphs of goethite (lepidocrocite and akageneite) would make for equally compelling studies as this would focus on evaluating the role crystal structure has in accommodating shock compression forces as opposed to the bond strength. Furthermore, this research builds off recent investigations into the shock resistance of cryptomelane (Murchland et al., 2024) and offers a timely comparative study. In cryptomelane, the Mn3+– O bond strength is ~ 402 kJ/mol which is comparable to the ~ 390 kJ/mol Fe3+– O bond strength in goethite. Both are elongated minerals with low density sites located within the crystal structure and both have shown resistance to dynamic shock compression. Further study on the shock resistant nature of other elongated, or fibrous, minerals such as sepiolite or palygorskite are recommended and should be compared with goethite and cryptomelane to further investigate the role that the thixotropic-like mechanism has for understanding material evolution within these contexts.
Conclusions
Initial analysis of synthetic goethite after dynamic shock compression experiments concluded that goethite displayed resistance to shock. TEM analysis revealed no change to the crystal morphology. Localized regions within individual goethite crystals show lattice fringe distortion and amorphous regions, predominantly located near crystal faces. Raman spectroscopy documented a noticeable shift in peaks between unshocked and shocked goethite indicating some transformations had likely occurred and the likely formation of hematite or a structurally similar iron oxide (e.g., magnetite). XRD indicates goethite, and possible magnetite, are common post shock phases. Owing to the inherent nature of the experimental materials and conditions, combined with the nature of the sample volumes associated with the analytical methods employed (TEM, Raman, XRD), some sample bias may have occurred. However, when all data sources are considered, goethite particles have persisted through shock.
Here a “thixotropic-like” model of collapse and relaxation of nanopores, or low-density areas, within goethite’s crystal structure is proposed. The shock resistant nature of goethite builds off a similar study of cryptomelane (Murchland et al., in review) and opens the door for exploring other similar minerals and their use across a variety of material science applications.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper.
References
Bassel F, Roques J, Gautheron C (2020) Neon diffusion in goethite, α-FeO(OH): a theoretical multi-scale study. Phys Chem Miner. https://doi.org/10.1007/s00269-020-01083-w
Basset WP, Johnson BP, Salvati LIII, Nissen EJ, Bhowmick M, Dlott D (2020) Shock initiation microscopy with high time and space resolution. Propell Explos, Pyrotech 45:1–14. https://doi.org/10.1002/prep.201900222
Bernal JD, Dasgupta DR, Mackay AL (1958) The oxides and hydroxides of iron and their structural interrelationships. Clay Miner Bull 4(21):15–30. https://doi.org/10.1180/claymin.1959.004.21.02
Bhowmick M, Nissen EJ, Dlott D (2018) Detonation on a tabletop: nitromethane with high time and space resolution. J Appl Phys 10(1063/1):5043540
Brugina EB, Kustova GN, Isupova LA, Tysbula SV, Kryukova GN, Sadykov VA (2000) Investigation of the structure of protohematite—metastable phase of Ferrum(III) oxide. J Mol Catal A Chem 158:257–261. https://doi.org/10.1016/S1381-1169(00)00086-8
Chao ECT (1967) Shock effects in certain rock forming minerals. Science 156:192–202. https://doi.org/10.1126/science.156.3772.192
Chen AA, Heaney PJ, Post JE, Fischer TB, Eng PJ, Stubbs JE (2021) Superhydrous hematite and goethite: a potential water reservoir in the red dust of Mars? Geology 49(11):1343–1347
Christensen PR et al (2000) Detection of crystalline hematite mineralization on Mars by the thermal emission spectrometer: evidence for near-surface water. J Geophys Res 105(E4):9623–9642. https://doi.org/10.1029/1999JE001093
Cornell RM, Schwertann U (2003) The iron oxides: structure, properties, reactions, occurrence, and uses, 2nd edn. Wiley-VCH Publishing
Cudennec Y, Lecerf A (2005) Topotactic transformations of goethite and lepidocrocite into hematite and maghemite. Solid State Sci 7(5):520–529. https://doi.org/10.1016/j.solidstatesciences.2005.02.002
Cymes B, Almquist CA, Krekeler MPS (2021) Effects of Mn(II) and Eu(III) cation exchange in sepiolite-titanium dioxide nanocomposites in the photocatalytic degradation of Orange G. ChemistrySelect 6:5180–5190. https://doi.org/10.1002/slct.202100303
Cymes BA, Almquist C, Krekeler MPS (2020) Europium-doped cryptomelane: multi-pathway synthesis, characterization, and evaluation for the gas phase catalytic oxidation of ethanol. Appl Cat A Gen 589:117310. https://doi.org/10.1016/j.apcata.2019.117310
De Faria DLA (1963) Dehydration of goethite and diaspore. Z Kristallogr 119(3–4):176–203. https://doi.org/10.1524/zkri.1963.119.3-4.176
De Faria DLA (1997) Raman microspectroscopy of some iron oxides and oxyhydroxides. J Raman Spectrosc 28(11):873–878. https://doi.org/10.1002/(SICI)1097-4555(199711)28:11%3c873::AID-JRS177%3e3.0.CO;2-B
Dlott D (2011) New developments in the physical chemistry of shock compression. Annu Rev Phys Chem 62:575–597. https://doi.org/10.1146/annurev.physchem.012809.103514
Dong H, Kukkadapu R, Fredrickson J, Zachara JM, Kennedy DW, Kostandarithes HM (2003) Microbial reduction of structural Fe(III) in illite and goethite. Environ Sci Technol 37(7):1268–1276. https://doi.org/10.1021/es020919d
Dunnwald J, Otto A (1989) An investigation of phase transitions in rust layers using raman spectroscopy. Corros Sci 29(9):1167–1176. https://doi.org/10.1016/0010-938X(89)90052-8
Fan H, Song B, Li Q (2006) Thermal behavior of goethite during transformation to hematite. Mater Chem Phys 98(1):148–153. https://doi.org/10.1016/j.matchemphys.2005.09.005
Forbes JW (2012) Shock wave compression of condensed matter. Springer, p 388
Gan B, Jiang G, Huang Y, Zhang H, Hu Q, Zhang Y (2023) Phase diagram and thermoelastic property of iron oxyhydroxide across the spin crossover under extreme conditions. Phys Rev B 107:064106. https://doi.org/10.1103/PhysRevB.107.064106
Gialanella S, Girardi F, Ischia G et al (2010) On the goethite to hematite phase transformation. J Thermal Anal Calorim 102:867–873. https://doi.org/10.1007/s10973-010-0756-2
Gleason AE, Jeanloz R, Kunz M (2008) Pressure-temperature stability studies of FeOOH using X-ray diffraction. Am Miner 93:1882–1885. https://doi.org/10.2138/am.2008.2942
Goss CJ (1987) The kinetics and reaction mechanisms of the goethite to hematite transformation. Mineral Mag 51(361):437–451. https://doi.org/10.1180/minmag.1987.051.361.1
Gualtieri AF, Venturelli P (1999) In situ study of the goethite-hematite phase transformation by real time synchrotron powder diffraction. Am Miner 84(5–6):895–904. https://doi.org/10.2138/am-1999-5-625
Hanesch M (2007) Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys J Int 177(3):941–948. https://doi.org/10.1111/j.1365-246X.2009.04122.x
Huguenin RL (1974) The formation of goethite and hydrated clay minerals on Mars. J Geophys Res 79(26):3895–3905. https://doi.org/10.1029/JB079i026p03895
Jia F, Ramirex-Muñez K, Song S (2015) Mechanism for the formation of micropores in the thermal decomposition of goethite to hematite. Surf Interface Anal 47(4):535–539. https://doi.org/10.1002/sia.5744
Klingelhöfer G et al (2004) Jarosite and hematite at Meridiani Planum from opportunity’s Mossbauer spectrometer. Science 306(5702):1740–1745. https://doi.org/10.1126/science.1104653
Kustova GN, Burgina EB, Sadykov VA, Poryaev SG (1992) Vibrational spectroscopic investigation of the goethite thermal decomposition products. Phys Chem Miner 18:379–382. https://doi.org/10.1007/BF00199419
Liu K, Dai L, Li H, Hu H, Zhuang Y, Yang L, Pu C, Hong M (2019) Pressure-induced phase transitions for goethite investigated by Raman spectroscopy and electrical conductivity. High Press Res 39:106–116. https://doi.org/10.1080/08957959.2019.1572751
Murchland M, Elasamar S, Viner G, Zhou X, Gillis M, Almquist C, Cymes B, Bhowmick M, McLeod CL, Krekeler MPS (2024)The effect of shock compression on the crystal structure of cryptomelane (K-OMS-2). J Dyn Behav Mat. https://doi.org/10.1007/s40870-023-00403-9
Mota e Nogueira V, Barbosa PF, Mayanna S, Silva AM, Toledo CLB, Lagoeiro LE, Assis LMD (2022) Characterization of the crystallographic preferred orientation relationships of the magnetite-hematite-goethite phase transformation during martitization. Minerals 12:326. https://doi.org/10.3390/min12030326
Nagai T, Kagi H, Yamanaka T (2003) Variation of hydrogen bonded O···O distances in goethite at high pressure. Am Miner 88:1423–1427. https://doi.org/10.2138/am-2003-1005
Nissen EJ, Bhowmick M, Dlott D (2021a) Shock-induced kinetics and cellular structures of liquid nitromethane detonation. Combust Flame 225:5–12. https://doi.org/10.1016/j.combustflame.2020.10.046
Nissen EJ, Bhowmick M, Dlott DD (2021b) Ethylenediamine catalyzes nitromethane shock-to-detonation in two distinct ways. J Phys Chem B 125:8185–8192. https://doi.org/10.1021/acs.jpcb.1c04427
Offield TW (1987) Shock effects in rocks and minerals. In: Structural geology and tectonics. Encyclopedia of Earth science. Springer, Berlin
Ramimoghadam D, Bagheri S, Abd Hamid SB (2014) Progress in electrochemical synthesis of magnetic iron oxide nanoparticles. J Magn Magn Mater 368:207–229. https://doi.org/10.1016/j.jmmm.2014.05.015
Rampe EB et al (2020) Mineralogy of vera rubin ridge from the mars science laboratory CheMin instrument. JGR Planets 125(9):e2019JE006306. https://doi.org/10.1029/2019JE006306
Ruan HD, Frost RL, Kloprogge JT (2001) The behavior of hydroxyl units of synthetic goethite and its dehydroxylated product hematite. Spectrochim Acta Part A Mol Biomol Spectrosc 57(13):2575–2586. https://doi.org/10.1016/S1386-1425(01)00445-0
Schwertmann U, Cornell RM (1991) Iron oxides in the laboratory. VCH Publishing
Schwertmann U, Taylor RM (1989) Iron oxides. Miner Soil Environ 1:379–438
Shim SH, Duffy T (2002) Raman spectroscopy of Fe2O3 to 62 GPa. Am Miner 87(2–3):318–326. https://doi.org/10.2138/am-2002-2-314
Short NM (1966) Shock processes in geology. J Geol Educ 14:149–166. https://doi.org/10.5408/0022-1368-XIV.4.149
Stekovic S, Springer HK, Bhowmick M, Dlott DD, Stewart DS (2021) Laser-driven flyer plate impact: computational studies guided by experiments. J Appl Phys 129:195901. https://doi.org/10.1063/5.0049817
Szytuta A, Burewicz A, Dimitrijevic Z, Krasnicki S, Rzany H, Todorovic J, Wanic A, Wolski W (1968) Neutron diffraction studies of a α-FeOOH. Phys Status Solidi 26:429–434. https://doi.org/10.1515/9783112496763-004
Tang R, Chen J, Zeng Q, Li Y, Liang X, Yang B, Wang Y (2020) Study on the high-pressure behavior of goethite up to 32 GPa using X-Ray diffraction, Raman, and electrical impedance spectroscopy. Minerals 10(2):99. https://doi.org/10.3390/min10020099
Wackerle J (1962) Shock-wave compression of quartz. J Appl Phys 33:922–937. https://doi.org/10.1063/1.1777192
Walter D, Buxbaum G, Laqua W (2001) The mechanism of the thermal transformation from goethite to hematite. J Therm Anal Calorim 63:733–741. https://doi.org/10.1023/A:1010187921227
Watari F, Delavignette P, Amelinckx S (1979) Electron microscopic study of dehydration transformations. II The formation of “superstructures” on the dehydration of goethite and diaspore. J Solid State Chem 29(3):417–427. https://doi.org/10.1016/0022-4596(79)90198-1
Waychunas G (1991) Crystal chemistry of oxides and oxyhydroxides. In: Lindsley DH (ed) Oxide minerals, vol 25. Mineralogical Society of America
Wells MA, Fitzpatrick RW, Gilkes RJ (2006) Thermal and mineral properties of Al-, Cr-, Mn-, Ni-, and Ti-substituted goethite. Clay Clay Miner 54(2):176–194. https://doi.org/10.1346/CCMN.2006.0540204
Williams Q, Guenther L (1996) Pressure induced changes in the bonding and orientation of hydrogen in FeOOH-goethite. Solid State Commun 100(2):105–109. https://doi.org/10.1016/0038-1098(96)00374-2
Wolska E (1981) The structure of hydrohematite. Z Für Kristallogr Cryst Mater 154:69–76. https://doi.org/10.1524/zkri.1981.154.14.69
Wolska E (1988) Relations between the existence of hydroxyl ions in the anionic sublattice of hematite and its infrared and X-ray characteristics. Solid State Ionics 28–30:1349–2135. https://doi.org/10.1016/0167-2738(88)90385-2
Zhang WJ, Huo CF, Feng G, Li YW, Wang J, Jiao H (2010) Dehydration of Goethite to hematite from molecular dynamics simulations. J Mol Struct (thoechem) 950(1–3):20–26. https://doi.org/10.1016/j.theochem.2010.03.013
Zhou X, Miao YR, Shaw W, Suslick K, Dlott D (2019) Shock wave energy absorption in metal-organic framework. J Am Chem Soc 141(6):2220–2223. https://doi.org/10.1021/jacs.8b12905
Zhou L, Jiang G, Gan B, Zhuang Y, Zhang H, Zhang Y (2023) Sound velocities of natural goethite across α–ɛ phase transformation under dynamic compression. J Appl Phys 133(14):145902. https://doi.org/10.1063/5.0136788
Zhu YQ, Sekine T, Kobayashi T, Takazawa E, Terrones M, Terrones H (1998) Collapsing carbon nanotubes and diamond formation under shock waves. Chem Phys Lett 287(5–6):689–693. https://doi.org/10.1016/S0009-2614(98)00226-7
Zhu YQ, Sekine T, Brigatti KS, Firth S, Tenne R, Rosentsveig R, Kroto HW, Walton DR (2003) Shock-wave resistance of WS2 nanotubes. J Am Chem Soc 125(5):1329–1333. https://doi.org/10.1021/ja021208i
Acknowledgements
We thank Dr. Dana Dlott at the University of Illinois at Champaign-Urbana for laboratory access. Mr. Matt Duley and Dr. Zachery Oestreicher of Miami University’s Center for Advanced Microscopy and Imaging (CAMI) for general facility assistance. Jenkins acknowledges a Graduate Assistantship from Miami University which supported him as this work was completed.
Author information
Authors and Affiliations
Contributions
NRJ executed the project as part of the fulfilment of his M.S. degree requirements and collected TEM data, XRD data, and interpreted data and contributed writing and editing on the M.S. thesis. XZ performed all shock experimental work and collected Raman spectroscopy data and contributed to significant writing and editing. MB contributed to planning and significant writing and editing. CMc contributed significant editing and writing. MPSK managed the project and contributed to significant writing and editing as well as instruction of Jenkins in analytical work.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jenkins, N.R., Zhou, X., Bhowmick, M. et al. Investigation into the stability of synthetic goethite after dynamic shock compression. Phys Chem Minerals 51, 22 (2024). https://doi.org/10.1007/s00269-024-01279-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-024-01279-4