Abstract
The crystal structure and bonding environment of K2Ca(CO3)2 bütschliite were probed under isothermal compression via Raman spectroscopy to 95 GPa and single crystal and powder X-ray diffraction to 12 and 68 GPa, respectively. A second order Birch-Murnaghan equation of state fit to the X-ray data yields a bulk modulus, \({K}_{0}=46.9\) GPa with an imposed value of \({K}_{0}^{\prime}= 4\) for the ambient pressure phase. Compression of bütschliite is highly anisotropic, with contraction along the c-axis accounting for most of the volume change. Bütschliite undergoes a phase transition to a monoclinic C2/m structure at around 6 GPa, mirroring polymorphism within isostructural borates. A fit to the compression data of the monoclinic phase yields \({V}_{0}=322.2\) Å3\(,\) \({K}_{0}=24.8\) GPa and \({K}_{0}^{\prime}=4.0\) using a third order fit; the ability to access different compression mechanisms gives rise to a more compressible material than the low-pressure phase. In particular, compression of the C2/m phase involves interlayer displacement and twisting of the [CO3] units, and an increase in coordination number of the K+ ion. Three more phase transitions, at ~ 28, 34, and 37 GPa occur based on the Raman spectra and powder diffraction data: these give rise to new [CO3] bonding environments within the structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rare carbonate mineral bütschliite (also spelled buetschliite), with chemical formula K2Ca(CO3)2, has been observed to form in highly disparate geological environments. First discovered and named in 1947, the mineral was initially found in wood-ash stones from the western United States (Milton and Axelrod 1947). More recently, bütschliite has been identified as an inclusion/daughter phase in mantle-derived olivine and diamond crystals (Abersteiner et al. 2022; Logvinova et al. 2019). Occurrences of bütschliite in granitic pegmatites have also been reported (Bermanec et al. 2011).
The identification of bütschliite inclusions not only in diamond, but in mantle-derived olivine as well, suggests that alkaline carbonate melts and solid mineral phases could play an important role in the petrogenesis of kimberlites and carbonatites. Indeed, Chayka et al. (2021) suggests that even alkaline-poor carbonatites may be derived from substantially alkaline-enriched parental magmas. Candidates for potassium-bearing mineral phases in the mantle are limited: the phyllosilicate mineral phlogopite and the amphibole richterite have been identified in a number of mantle-derived xenoliths and within the results of experiments on hydrated and carbonated peridotite (e.g., Harlow and Davies 2004; Meltzer and Kessler 2023), but in terms of potassic carbonate minerals, only bütschliite has been reported in mantle assemblages. Several studies have explored the stability of the related synthetic material K2Mg(CO3)2 (Brey et al. 2011; Golubkova et al. 2015; Arefiev et al. 2018), but this compound has not been identified as a mineral phase in nature.
Investigations into the stability and structure of bütschliite at non-ambient conditions have been limited. McKie (1990) studied the system K2Ca(CO3)2–Na2Mg(CO3)2 and determined that fairchildite, the intrinsically-disordered high-temperature polymorph of bütschliite, and eitelite (Na2Mg(CO3)2) can form an extensive solid solution, while bütchsliite and eitelite are almost completely immiscible (limited to < 2 mol% solid solution). Arefiev et al. (2019) and Shatskiy et al. (2015) studied the K2CO3-CaCO3 system at high temperatures and 3 and 6 GPa, respectively, and observed the presence of bütschliite as a sub-solidus phase at each pressure. Hou et al. (2022) and Tian et al. (2023) employed first-principles simulations via the VASP calculational package (Kresse and Furthmüller 1996) to examine the structure of bütschliite under isothermal compression to ~ 22 and ~ 26 GPa, respectively, and both studies calculated transitions to monoclinic and triclinic phases, albeit with different sequences and at different pressures. Zhuravlev (2022) deployed the CRYSTAL17 ab initio package to calculate the elastic constants of bütschliite under compression. He noted that the C44 shear elastic constant appeared to be soft, trending towards zero at 5.8 GPa, thus indicating that an unspecified phase transition is anticipated at this pressure. In this study, we present the results of the first in-situ high-pressure, ambient-temperature Raman spectroscopy and synchrotron X-ray diffraction experiments on bütschliite to 95 GPa. These delineate the transition pressures and properties of the high-pressure phases of this material. Our results shed light on the crystal structures and bonding environments of low symmetry carbonate minerals at high pressures, as well as the role played by cations in the compressibilities of these minerals.
Methods and sample synthesis
Bütschliite crystals were synthesized following the method of Pabst (1974), in which millimeter-scale cleavage rhombs of optical-quality calcite are immersed in a saturated K2CO3 solution and heated to between 50 and 70°C for several days. Crystals grown in this manner are typically euhedral and free of inclusions, making them suitable for both optical and X-ray-based measurements. While bütschliite was the most abundant phase produced by this method, small amounts of other (possibly hydrated) potassium/calcium carbonate phases were sporadically detected in Raman spectroscopy measurements. Bütschliite is hygroscopic and deliquesces readily in air over the course of a few hours. Samples were stored in toluene after synthesis; crystals stored in this organic liquid remain stable indefinitely. As a mildly non-hydrostatic pressure medium, silicone oil was chosen for some experiments over a methanol/ethanol solution to minimize the risk of crystal degradation due to hydration; neon, which more closely approaches hydrostaticity at high pressures, was used as a pressure medium for a large number of the experiments. Notably, crystals stored in high purity methanol/ethanol solutions (< 0.01% water) at slightly elevated pressure did not show any signs of degradation over the course of a few weeks.
Raman spectroscopy
Ambient and high-pressure Raman spectra were collected on a LabRAM HR Evolution spectrometer equipped with a 532 nm excitation laser nominally rated at 50 mW. A grating of 1200 lines/mm was employed and spectral resolution is ~ 1 cm−1. Samples were loaded into symmetric-style diamond anvil cells equipped with 250 or 350 μm culet diamonds. Rhenium gaskets were used for all Raman experiments, and silicone oil or neon were used as pressure transmitting media in different runs. Ruby spheres or small amounts of ruby powder were loaded into the sample chamber and the calibration of Shen et al. (2020) was used for pressure determination. Ruby emission lines became unresolvable at pressures above ~ 85 GPa, and pressures were determined from the Raman shift of the diamond anvil measured at the center of the culet (Akahama and Kawamura 2006). Peak fitting and deconvolution were carried out with the Horiba LabSpec 6 software.
Synchrotron single crystal X-ray diffraction
The high-pressure single-crystal X-ray diffraction experiments were performed at ambient temperature using short symmetric, Merrill-Bassett, and BX-90 diamond anvil cells equipped with 300–400 µm culet Boehler-Almax diamond anvils and c-BN/WC seats providing a ~ 90° opening angle. Re and Inconel gaskets were indented to ~ 30–50 µm, and sample chambers were drilled with diameters of 150–180 µm with a laser-milling system. Single-crystal samples, measuring ~ 50 × 50 × 10 µm, were mounted directly onto the diamond culet with a small amount of vacuum grease to minimize movement of the crystal during loading of the pressure media. Neon or silicone oil were used as pressure transmitting media in different runs. Pressure media were loaded into the sample chambers immediately after sample loading to avoid the risk of sample degradation.
The high-pressure diffraction data were collected at end-station 2 of beamline 12.2.2 of the Advanced Light Source at Lawrence Berkeley National Laboratory using synchrotron X-rays monochromated by silicon (111) with an energy of 30 keV (\(\lambda =0.4132(1)\) Å), focused to a 10 × 10 μm spot. The diffraction spots were collected on a Pilatus3 S 1 M fast detector. Exposure times ranged from 1 to 8 s/º. Detector distance and wavelength calibration were performed using CeO2 powder and a NIST single-crystal ruby sphere. The sample was aligned to the rotational center of the goniometer, and data were collected throughout the range of \(\theta =-35^\circ\) to \(35^\circ\) (in \(0.50^\circ\) and \(1.00^\circ\) increments) for a total coverage of ~\(70^\circ\) sample rotation. The diffraction data were corrected for the reduction of reflection intensities due to the DAC-gasket shadowing and indexed and reduced to hkl sorted structure factors by integration using the program SAINT v8.34A in APEX3 (Bruker 2016). Before structure solution steps, a few individual reflections (with negative and almost zero intensities) were removed from the data set because their intensities were affected by simultaneous diffraction events in the diamond anvils. A total of ~ 150–250 unique reflections were available for subsequent structural analysis. The structure solutions were carried out with SHELXS (embedded in APEX) using direct methods and refined with SHELXT (Sheldrick 2008) by full-matrix least-squares on F2. For every structure, ~ 34 parameters including overall scale factor, isotropic atomic displacement factors, and fractional coordinates of the atoms were refined; for bütschliite-II, the greater number of distinguishable atoms within the unit cell increased the number of parameters solved. Ball and stick structural models were produced in VESTA 3 (Momma and Izumi 2011).
Synchrotron powder X-ray diffraction
High-pressure powder X-ray diffraction measurements were also collected at ALS beamline 12.2.2, using BX-90, symmetric, and Merrill-Bassett diamond anvil cells with diamond culet sizes ranging between 300 and 500 μm. Diamonds were mounted on c-BN or WC seats. Inconel and rhenium foils were used for gasket materials, and silicone oil or neon were employed as pressure media in different runs. An exposure time of 30 s was used and X-ray beam energies of 25 and 30 keV were used in different runs. Powder patterns were indexed with the LeBail method in GSAS-II (Toby and Von Dreele 2013). Peterson (2005) suggests that the LeBail method can be inappropriate for low symmetry phases; however, this method was preferable to the Rietveld method for these samples since the acquired powder patterns were highly textured due to apparent preferred orientation of grains of the first high pressure phase.
Results and discussion
Single crystal and powder X-ray diffraction of bütschliite
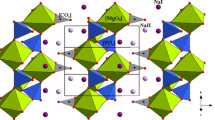
X-ray diffraction measurements were previously made on bütschliite-I under ambient conditions. Mrose et al. (1966) erroneously reported, due to a misprint in the original publication, that bütschliite crystallizes in the \(R\overline{3 }\) space group. Pabst (1974) determined that bütschliite crystallizes in the \(R\overline{3 }m\) space group (reported as \(R\overline{3 }2/3\)) with a = 5.38 Å, c = 18.12 Å, and Z = 3, and has a structure very similar to that of eitelite, despite the difference in space groups (eitelite belongs to the \(R\overline{3 }\) space group and lacks a mirror plane due to rotation of the carbonate anions). The ambient bütschliite structure is characterized by planar sheets of [CO3] units separated by alternating interstitial layers of Ca2+ and K+ cations (Fig. 1a, b). Pabst (1974) reports a unit cell volume of 454.2 Å3, which is in excellent agreement with our measured value of 453.9 Å3. Compression of bütschliite is strongly anisotropic, with contraction of the c-axis accounting for most of the volume change (Fig. 2a–c). The contraction of the unit cell along the c-axis follows a steeply linear trend up to the first phase transition, with \(\Delta c\)/\(\Delta P\) = 0.23 Å/GPa. This style of anisotropy has also been observed in the isostructural synthetic compound K2Mg(CO3)2, and is a result of the greater compressibility of the [KO9] polyhedra compared to the alkaline earth cation polyhedra and trigonal planar [CO3] units (Golubkova et al. 2015). Most of the reduction in the bütschliite unit cell volume occurs via reductions of the K–Ca and K–O distances. The C–O, O–O, and Ca–O distances remain largely unchanged during compression (Fig. 1).
Structural refinements of bütschliite and bütschliite-II at 0 and 7.6 GPa, respectively, from different perspectives (a–e). Atomic spacings of bütschliite and bütschliite-II derived from single-crystal measurements (f–k). Note that the structures in (c–e) are drawn with the K+ ions in 12-fold coordination. The unfilled square symbols in (h) show distances between unbonded potassium and oxygen atoms that become bonded after the phase transition
Lattice parameters (a–c), \(\beta\)-angles (d), formula unit normalized volumes (e), and unit cell volumes of bütschliite and bütschliite-II during compression (f). The green markers in (c) show the \(3c{\text{sin}}(\beta )\) trend, which is directly comparable to the c-axis in the low-pressure phase. The black star in (f) is the calculated zero-pressure volume of bütschliite-II from the method of Jeanloz (1981). Equation of state parameters for (f) are reported in the text. Fits only include the experimental X-ray data. The data point of Hou et al. (2022) at 10 GPa is in a metastable regime of the C2/m phase: they calculate that the \(P\overline{1 }\) structure is stable from 3.3 to 10.3 GPa. Tian et al. (2023) do not report lattice parameters or \(\beta\)-angles; the C2/m phase is stable in their calculations from either 3.3 or 3.5 GPa to 10.3 or 10.5 GPa (their paper is inconsistent in its reporting of the pressure of transitions). Error bars fall within the symbols
The combined single crystal and powder pressure–volume data were recast into Eulerian stress–strain coordinates and fit with a Birch-Murnaghan equation of state (Figure SI 1). The equation of state was fit to the stress–strain data using an orthogonal distance regression to account for uncertainties in both pressure and volume resulting in a bulk modulus of \({K}_{0}=46.9(17)\) GPa with an imposed value of \({K}_{0}^{\prime}=4\) (Fig. 2f). The equation of state fit to the X-ray diffraction data indicates that the structure is more compressible than the results from the first principles calculations of Hou et al. (2022), but close to the results of Zhuravlev (2022). Our values of \({K}_{0}\) are 23% smaller than the value calculated by Hou et al. (2022). Notably, our measurements of the bütschliite low-pressure phase extend to higher pressures than the calculations of Hou et al. (2022), as their calculations yield a \(P\overline{1 }\) phase intermediate between the \(R\overline{3 }m\) and C2/m phases at pressures between 3.3 and 10.3 GPa: this phase is not seen in the X-ray diffraction data in this pressure range. In terms of elasticity, the discrepancy between the theory and experiment appears to primarily involve the compressibility of the c-axis: the calculations of Hou et al. (2022) underpredict the experimentally observed compressibility of this axis.
Single crystal and powder X-ray diffraction of bütschliite-II
Bütschliite undergoes a phase transition between 5.5 and 6.0 GPa. Our lowest pressure refinement of the bütschliite-II phase from the single crystal data is at 6.3 GPa. The high-pressure phase crystallizes in the monoclinic system with C2/m symmetry (space group #12) and Z = 2, with cell parameters at this pressure of a = 9.182(7) Å, b = 5.299(6) Å, c = 6.354(11) Å, and \(\beta =120.06(3)^\circ\). The volume change across the phase transition on a formula unit basis is small but discontinuous, indicating that the transition is first order (Fig. 2e). This is in accord with hysteresis observed in the transition using Raman spectroscopy, described below. Based on the single-crystal data, there is a 2% density increase associated with the transition from the hexagonal to monoclinic phase between 5.5 and 6.3 GPa. The small \(\Delta V\) across the phase transition indicates that the Clapeyron slope between the two phases is likely to be relatively flat, which in turn suggests that the pressure of the phase transition is unlikely to have a robust temperature dependence. The transition from the hexagonal to monoclinic phase is reversible, with the high-pressure phase reverting to bütschliite on decompression below ~ 2 GPa. Following the method of Jeanloz (1981), an equation of state was fit to the high-pressure bütschliite-II phase (Figure SI 2) with parameters \({V}_{0}=322.2\) Å3, \({K}_{0}=24.8(44)\) GPa, and \({K}_{0}^{\prime}=4.0(11)\) (Fig. 2f). Our fit bulk modulus is 50% smaller than the value calculated by Hou et al. (2022). And, the bulk modulus of bütschliite-II is substantially less than the bulk modulus of K2Mg(CO3)2-II, \({K}_{0}=58.4\) GPa, reported by Golubkova et al. (2015). The bulk moduli of the bütschliite-I and -II phases are low relative to most other carbonates, but that of the low-pressure phase is comparable to the bulk moduli of BaCO3-witherite and Ag2CO3 (Wang et al. 2015; Santamaria-Perez et al. 2023). Similarly, the lower bulk modulus of the high-pressure phase is unusual, but not unprecedented for carbonates: a high-pressure phase in layered Ag2CO3 has a bulk modulus of 26 GPa (Santamaria-Perez et al. 2023). A key aspect here is that the C2/m symmetry phase appears to be stabilized at high pressures by its high compressibility.
Notably, there is not particularly good agreement between the volumes and phase transition pressures of the theoretical calculations and experiments plotted in Fig. 2. The first principles simulations of Hou et al. (2022) and Tian et al. (2023), carried out at 0 K, both predict phase transitions near 3.5 and 10.5 GPa, but the order of the transitions is different. Hou et al. (2022) report a transition from the \(R\overline{3 }m\) phase to a triclinic \(P\overline{1 }\) structure at 3.3 GPa followed by a transition to the monoclinic C2/m structure at 10.3 GPa, while Tian et al. (2023) report a transition to the monoclinic C2/m structure at 3.3–3.5 GPa and a transition to the triclinic \(P\overline{1 }\) structure at 10.3–10.5 GPa. The source of this disparity is unclear, as both simulations were conducted using the VASP software package, and the cut-off and convergence criteria of their calculations seem to be essentially identical. That said, the reported differences in enthalpies between the three phases at 0 K are quite small: near 10 GPa, less than 0.08 eV/formula unit reported by Hou et al. (2022), and less than 0.07 eV/atom according to Tian et al. (2023). The difference between the C2/m and the \(P\overline{1 }\) structures is even smaller, being less than 0.01 eV/formula unit (Hou et al. 2022) or 0.01 eV/atom (Tian et al. 2023) up to 12 GPa. As such, calculations at 0 K without thermal corrections might not accurately predict phase stabilities for such energetically close phases even at ambient temperatures. For comparison, Zhuravlev (2022) (using a different ab initio software package) predicts a shear instability to an unspecified structure at 5.8 GPa. There is also likely an effect of kinetics on the experimentally observed bütschliite-I to -II phase transition at 300 K: the transition is observed on the up-stroke near 6 GPa, and the reversion is observed on the down-stroke near 2 GPa. Accordingly, the equilibrium transition may occur close to 4 GPa (from the mid-point between the up-stroke and down-stroke transition pressures) at 300 K. Consideration of kinetic effects thus might somewhat improves the agreement between theory and experiment with respect to the pressure of the first transition in this material.
In contrast to the low-pressure phase, where compression occurs predominantly along the c-axis of the hexagonal cell, rotation and interlayer shifting seem to be almost entirely responsible for the enhanced compressibility of the bütschliite-II phase. The trend of \(3c{\text{sin}}(\beta )\) in Fig. 2c serves as an analogue for the interlayer spacing within the low-pressure phase, and shows that the interlayer compression is largely monotonic across the transition. The change in slope of the \(3c{\text{sin}}(\beta )\) trend is likely a result of changing K–O bond lengths (discussed below). All axial parameters follow relatively flat linear trends on compression while the \(\beta\)-angles follow an increasing quadratic trend, with \(\beta \left(P\right)=-0.033{P}^{2}+1.371P+113.204\) (Fig. 2d). This increase in \(\beta\)-angle directly reflects the pressure-induced lateral shifting between layers (e.g., Fig. 1d). It is this interlayer shifting that likely renders the high-pressure phase elastically softer than the low-pressure bütschliite phase: the complementary effects of carbonate group rotation and layer shifting provide additional means of compaction that are unavailable to the low-pressure phase. Beyond the rotation and layer shifting, the overarching structural similarities of the two phases produce pressure-induced trends of bond valence sums (Kunz and Brown 1995) that appear continuous between the two phases under compression: both cations and the oxygen ions have bond valence sums that increase over the pressure range of our single-crystal measurements (Figure SI 3).
The phase transition to bütschliite-II results in the bifurcation of several atomic distances and bond lengths (Fig. 1f, g, j, k). The high-pressure phase gives rise to two unique C–O and Ca–O bond lengths. The evolution of the K–O bond lengths is more complex. Within both the bütschliite and C2/m phases, potassium has been generally viewed as being in nine-fold coordination: in the C2/m phase, the 9-coordinate geometry is that of a distorted tri-capped trigonal prism (e.g., Akella and Keszler 1995a, b). However, within the K2Ca(CO3)2 C2/m phase, depending on the radius of the coordination sphere of potassium, the K+ cations may be viewed as undergoing a coordination change, initially forming elongated [KO12] cuboctahedra (perhaps more appropriately viewed as 9 + 3 coordination), with the longer K–O bond lengths being coupled to the rotation of the [CO3] anions (Fig. 1h). Here, we view K–O distances of 3.3 Å and less as being clearly within the coordination sphere of the K ion, based on the maximum eight-fold K–O distances in a survey of K-bearing compounds being 3.17 Å (Wood and Palenik 1999), and the typical difference in bond distance between eight and 12-fold coordination being 0.13 Å (Shannon 1976). Such distances are accessed by additional oxygens above ~ 8 GPa (Fig. 1h); the general trajectory of the more distant K–O distances (Fig. 1h) indicates that this transition to higher coordination is progressive following the transition to the high pressure phase (with those distances closer than ~ 3.5–3.6 Å potentially being viewed as part of a secondary coordination distance, giving rise to 9 + 2 or 9 + 3 coordination).
With increasing compression, the [CO3] units become more oblique with respect to the a-axis and, as shown in Fig. 3, there is a direct line-of-sight interaction between the K+ ion and the neighboring carbon ion that is coordinated to the three additional oxygens that approach the coordination sphere of the potassium. Repulsion between the C and K atoms likely contributes to the rotation of the [CO3] units, as the C–K distance remains constant around 3.25 Å during compression: increasing pressure does, however, induce a rotation of the carbonate units. This shift in geometry rotates two oxygens closer to the potassium ion, and one further away: as such, at 9.5 GPa, the local coordination of potassium might be viewed as 11-fold, or 9 + 2 coordinated. Notably, if the C–K distance is indeed near some minimum critical value, then the rotation of [CO3] units and change in \(\beta\)-angle can be interpreted as structural distortions which serve to maintain this value.
We quantify the degree of tilting of the [CO3] units by measuring the angle \(\theta\), formed by the intersection of the (001) plane with a plane passing through the three oxygens atoms of the [CO3] unit, as a function of pressure. This trend is well characterized by a quadratic polynomial in pressure, with \(\theta (P)=-66.86+15.95P-0.79{P}^{2}\) (Fig. 4).
Tilt of [CO3] units in the bütschliite-II phase as a function of pressure, derived from single-crystal XRD refinements. The angle \(\theta\) is formed by the intersection of the (001) plane with a plane passing through the three oxygen atoms in a [CO3] unit. The fit is a quadratic fit intended to guide the eye
Our powder diffraction data on bütschliite access considerably higher pressure conditions, up to 67.5 GPa (Figure SI 4). These data show strong evidence for multiple higher pressure phase transitions, for which the pressures of occurrence are more accurately determined by Raman spectroscopy. Nevertheless, clear evidence is seen for three transitions between ~ 26 and 40 GPa, although indexing of these phases proved challenging due to poor crystallinity/weak diffraction patterns, as well as probable preferred grain orientation in the high-pressure phases. New diffraction peaks associated with these phases are shown by arrows in Figure SI 4b. A key aspect here is that there is continuity of several features in the diffraction patterns throughout the pressure range to 67.5 GPa (Figure SI 4). This observation provides a general indication that the overall layered topology of the structure persists, with moderate symmetry changes, to the highest pressures of these measurements at 300 K.
Raman spectroscopy overview
Our ambient spectrum of the synthesized bütschliite (Fig. 5) is in good agreement with previous measurements (Arefiev et al. 2019) and calculations (Caracas and Bobocioiu 2011).
Representative spectra taken on compression up to 94 GPa, along with mode shifts, are plotted in Fig. 6. Our Raman measurements access substantially higher pressures than the XRD measurements, and we documented multiple phase transitions at elevated pressures in detail in the Raman spectral experiments which are discussed in the following sections.
Mode shifts (a–b) and representative spectra (c–f) of bütschliite on compression to 94 GPa. The first appearances of new phases are marked by lines at 6.0, 27.8, 33.5, and 37.4 GPa and the colors correspond to different phase regimes: bütschliite-I is black, bütschliite-II is green, bütschliite-III is orange, bütschliite-IV is magenta, and bütschliite-V is blue. For clarity, the largest peak intensities have been normalized to the same amplitude across panels c–f
Raman spectra of bütschliite-I
Factor group analysis of bütschliite-I yields
In this irreducible representation, the A1g and Eg modes are Raman active. We observe 10 peaks in the ambient bütschliite spectrum (Fig. 5), with eight first order (5Eg, 3A1g) and two second order modes (assignments are derived from Caracas and Bobocioiu 2011): six of these peaks are resolvable once the sample is loaded into the DAC with a pressure medium.
All observed modes exhibit positive linear frequency shifts with pressure up to the first phase transition to the C2/m structured phase (Fig. 7; Table 1). The symmetric (\({\nu }_{1}\)) and asymmetric (\({\nu }_{3}\)) stretches have similar pressure shift rates: 5.44 and 5.78 cm−1/GPa, respectively. The in-plane bend shifts at a considerably slower rate, with a value of 2.48 cm−1/GPa.
The lattice modes exhibit larger variations in their pressure shifts and are, based on their mode-Grüneisen parameters, more anharmonic. The A1g lattice mode with an initial frequency of 224 cm−1 shows the largest deviation from harmonicity among all observed modes: the large pressure-shift of this mode, which is associated predominantly with translations of the carbonate group along the c-axis of the structure (Caracas and Bobocioiu 2011), likely directly reflects the large compressibility of the c-axis observed within the diffraction experiments. Although the lack of thermal expansion data on bütschliite precludes calculation of a bulk thermodynamic Grüneisen parameter for this phase, it is clear from Table 1 that the largest contributors to this parameter are, as expected, among the lattice vibrations.
Raman spectra of bütschliite-II
The first signs of a phase transition observed in the Raman data occur at 6.5 GPa, and the spectrum at this pressure likely corresponds to a mixed-phase regime. At this pressure, the first hint of three new lattice modes appears between 110 and 150 cm−1. The highest frequency lattice mode broadens asymmetrically prior to splitting into three peaks, and \({\nu }_{4}\) and \({\nu }_{3}\) broaden asymmetrically prior to splitting into two peaks each. By 7.2 GPa the sample appears to be fully converted to the bütschliite-II phase. While the \({\nu }_{2}\) vibration is weakly present in the ambient bütschliite spectrum, it becomes, for the most part, unresolvable once the sample is loaded into the DAC with a pressure medium. The change in symmetry to C2/m in the high-pressure phase results in a substantial increase in the amplitude of the \({\nu }_{2}\) peak during compression (Fig. 8b).
Factor group analysis of the bütschliite-II structure yields
Here, the Ag and Bg modes are Raman active. Symmetry assignments and pressure shifts of the bütschliite-II carbonate modes are reported in Table 2 and Fig. 9. The mode Grüneisen parameters are calculated at high pressure using a Taylor expansion of \(K(P)\) with a truncating value of \({K}_{0}^{{\prime}{\prime}}\) (i.e., \({K}_{0}^{{\prime}{\prime}}=\frac{1}{9{K}_{0}}\left[63{K}_{0}^{\prime}-9{K}_{0}^{{\prime}2}-143\right]\): Birch 1978). The frequency shifts with pressure of all modes are positive, except for the \({\nu }_{2}\) and \(2{\nu }_{2}\) vibrations (the CO3 out-of-plane bend and its overtone). The negative frequency shift of the \({\nu }_{2}\) mode during compression has previously been explained for the aragonite structure (Pmcn symmetry) as resulting from the dominance of a negative force constant, produced by coupling between neighboring carbonate anions, over the positive force constant associated with the bending vibration of an isolated carbonate ion during compression (Decius 1955; Kraft et al. 1991).
Negative frequency shifts of the \({\nu }_{2}\) or 2 \({\nu }_{2}\) mode during compression have also been observed in a range of carbonate minerals, including calcite, strontianite, magnesite, dolomite, shortite, and bästnasite (Gillet et al. 1993; Vennari and Williams 2018, 2019; Bayarjargal et al. 2018; Vennari et al. 2018; Efthimiopoulos et al. 2019). The trajectory of the \({\nu }_{2}\) mode toward lower frequencies upon compression has substantial consequences for the evolution of the bütschliite spectrum, contributing to the development of a complex resonance phenomenon between the \({\nu }_{2}\) and \({\nu }_{4}\) vibrations observed at higher pressures.
The spectrum of bütschliite-II is remarkably similar to the ambient-pressure spectrum of Sr2Mg(BO3)2 presented by Lv et al. (2018), which also belongs to the C2/m space group, providing independent confirmation of our single-crystal refinements. To semi-quantitatively compare the spectra of the borate relative to the carbonate in Sr2Mg(BO3)2 and K2Ca(CO3)2, we use the quantity
where A and B are bonded elements, Z is the charge, r is the bond length (with values taken from Shannon 1976), and \(\mu ={m}_{A}{m}_{b}/({m}_{A}+{m}_{B})\) is the reduced mass. This relation is simply derived from Coulombic interactions coupled with harmonic oscillators. For the anions under consideration, \({\xi }_{B-O}=0.61\) and \({\xi }_{C-O}=0.75\). The ratio of these quantities,
serves as a scaling factor with \(\chi =1.23\). Application of this scaling factor to the ambient pressure fits of the bütschliite-II carbonate modes recovers the borate mode frequencies of Sr2Mg(BO3)2 to within 0.2 to 7.5%, with the largest deviation being for the asymmetric stretching vibration of the carbonate group (\({\nu }_{3}\)), which is anticipated to be substantially affected by the surrounding cations.
The spectra of bütschliite-II also show some novel spectroscopic features (Fig. 10). Specifically, Fermi resonance occurs when the wave functions of two vibrational modes with the same symmetry and similar energies mix. In vibrational spectra, the phenomenon manifests as the frequencies of two resonating peaks, \({\nu }_{-}\) and \({\nu }_{+}\), following a hyperbolic trajectory and exchanging intensity. We observed a Fermi resonance in the lattice modes of bütschliite-II, spanning from 9 to 29 GPa (Fig. 10). By comparing the spectra of the bütschliite-II with the calculated spectrum of Sr2Mg(BO3)2 from Lv et al. (2018), we are able to infer that the resonating modes each have Ag symmetry and involve vibrations of K+ against [CO3] units. The coupling constant, representing the semi major axis of the hyperbola in pressure-frequency space, is \(\delta\) = 9.27 cm−1 and the maximum resonance occurs at 19.7 GPa. Following Shimizu (1985), we calculated the unperturbed frequencies \({\nu }_{a}\) and \({\nu }_{b}\) (Fig. 10b), which represent the frequency trajectories the peaks would follow in the absence of resonance:
A notable feature of the bütschliite-II Fermi resonance is the nearly linear trajectory of the high frequency resonating peak, \({\nu }_{+}\) (Fig. 10b). Despite the asymmetric trajectories of the resonating peaks, the diagnostic intensity exchange between the peaks is clearly present over the duration of the resonance (Fig. 10c). The resonating modes, and indeed all Raman-active lattice modes in the C2/m phase, only involve motion of the carbonate and potassium ions. The \({\nu }_{+}\) vibration involves intralayer motion, while the \({\nu }_{-}\) vibration involves interlayer stretching and is dominated by the K–CO3 motion mostly along the c-axis. This axial direction is more compressible than the direction associated with the vibration in the ab-plane of \({\nu }_{+}\), resulting in the pronounced stiffening of the \({\nu }_{-}\) mode during compression. Hence, this resonance provides separate confirmation of the relative compressibilities of the different axes of the crystal that were characterized using single-crystal diffraction, and verifies that these structural trends persist to substantially higher pressures than the single-crystal data probe.
Subsequent phase transitions
We observe three more phase transitions in the Raman spectra, which are also manifested in the powder diffraction data (Figure SI 4): because of their narrow spacing in pressure, the Raman data provide better constraints on the transition pressures. There are two transitions to phases with limited stability ranges, bütschliite-III and bütschliite-IV, followed by a transition to bütschliite-V, which appears to be stable up to our highest-pressure measurement at 94.1 GPa. The first indications of a transition from bütschliite-II to bütschliite-III occur at 27.8 GPa, with the appearance of a low-frequency peak associated with the \({\nu }_{1}\) vibration at 1179 cm−1 (Fig. 11c). In the next pressure step, at 28.9 GPa (shown in Fig. 11c), the lattice modes broaden and decrease in intensity, a new high-frequency component associated with the \({\nu }_{2}\) vibration grows in at 898 cm−1, the low-frequency component of the \({\nu }_{1}\) vibration increases in intensity, and high-frequency components of both \({\nu }_{3}\) peaks appear. The \({\nu }_{1}\) peak of bütschliite-II loses intensity with increasing pressure and is fully absent by 35.2 GPa. Hence, the increase in band multiplicity of the \({\nu }_{2}\) and \({\nu }_{3}\) peaks is compatible with an expanded unit cell and/or a larger number of distinct carbonate groups within bütschliite-III relative to the C2/m structured bütschliite-II. This inferred decrease in symmetry is supported by the X-ray data, which show a bifurcation of the d-spacing associated with the (20 \(\overline{2 }\)) plane at ~ 26 GPa (Figure SI 4a). The lower frequency of the symmetric stretch (\({\nu }_{1}\)) in bütschliite-III might be associated with a lower force constant/longer distance of the C-O bonds, while the higher frequency components of the asymmetric stretches (\({\nu }_{3}\)) might indicate a stronger interaction with neighboring cations (possibly within a subset of carbonate sites). The net decrease in symmetric stretching (\({\nu }_{1}\)) frequency at this transition is roughly 15 cm−1; this is a substantially smaller decrement than that associated with the onset of 3 + 1 coordination of carbonate groups (Vennari and Williams 2018; Vennari et al. 2018), which generates a decrement of ~ 50 cm−1. This could be the triclinic \(P\overline{1 }\) structure predicted by Hou et al. (2022) to occur at 3.3 GPa, and Tian et al. (2023) to become stable at 10.3 or 10.5 GPa. While we do not preclude this possibility, it is notable that Hou et al. (2022) predicts essentially constant C–O distances between the C2/m and \(P\overline{1 }\) phases, which does not seem fully consistent with the change in the frequency of the symmetric stretch (\({\nu }_{1}\)) that we observe. In passing, we note that the clear doublet associated with the \({\nu }_{4}\) vibrations likely precludes this phase from being associated with the nyererite (Na2Ca(CO3)2) structure: only a single \({\nu }_{4}\) band is observed in this structure (Vennari et al. 2018; Rashchenko et al. 2017).
Raman spectra showing phase transitions from bütschliite-II through bütschliite-V. For clarity, the largest peak intensities have been normalized to the same amplitude across the panels. Unlabeled spectra were measured with the sample in a Ne PTM. Bütschliite-II is shown in green, bütschliite-III in orange, bütschliite-IV in magenta, and bütschliite-V in blue
The transition to bütschliite-IV occurs around 30.4 GPa in Ne. The onset of this phase is marked by the concomitant splitting of the \({\nu }_{2}\) vibration into a triplet and of the \({\nu }_{3}\) vibration into a multiplet of peaks, as well as a bifurcation of the (11 \(\overline{1 }\)) spacing in the X-ray data at ~ 32 GPa (Figure SI 4a). There is also an increase in the intensity of the lattice mode around 400 cm−1. By 35.2 GPa only a hint of the lowest frequency peak of the \({\nu }_{2}\) triplet remains. At 38.4 GPa \({\nu }_{3}\) reverts back to two peaks and the low frequency peak associated with \({\nu }_{4}\) starts to broaden. By 39.3 GPa, the components of the \({\nu }_{4}\) doublet have become substantially asymmetric. In this instance, the transition is relatively subtle: no major shifts in vibrational bands occurs, but there are changes in band multiplicities, with new bands being observed.
The transition to bütschliite-V is more dramatic, and occurs between 37.4 and 39.7 GPa in silicone oil and above 39.3 GPa in Ne. At 45.3 GPa, \({\nu }_{1}\) has split into several peaks (with at least two new components emerging on its low frequency side), and the \({\nu }_{4}\) and \({\nu }_{2}\) peaks become significantly broadened and distorted. Splitting of the \({\nu }_{1}\) vibration indicates the presence of multiple (possibly 4) distinct carbonate units within the unit cell, and the lowered frequency of these bands implies that a portion of the C–O bonds in the structure have weakened. The \({\nu }_{3}\) vibration becomes unresolvable beyond 50.6 GPa. The convergent trajectories of the \({\nu }_{2}\) and \({\nu }_{4}\) vibrations result in a complex resonance phenomenon that initiates at around 70 GPa (Fig. 6b).
The separation between the \({\nu }_{2}\) and \({\nu }_{4}\) vibrations vanishes near 70 GPa, and the coalesced peaks move together as a single unit upon further compression. While there is no evidence to suggest that the wave functions of the two vibrations are mixing, the energy of the vibrations is clearly becoming similar. In effect, the in-plane and out-of-plane bending vibrations of the carbonate unit have become energetically identical, and components associated with the two types of molecular vibrations span across a frequency range approaching 150 cm−1. Given the breadth of this bending region, it is certainly possible that combination bands of lattice modes as well as possible difference modes may be resonance enhanced by the bending vibrations, contributing minor, and possibly broad, peaks to this spectral region. A similar phenomenon was observed in dolomite-III (Vennari and Williams 2018) at similar pressures, and could possibly be an intrinsic feature of low-symmetry double carbonates subjected to very large compressions.
Notably, there is no indication of an increase in coordination associated with the carbonate group towards a 3 + 1 coordination, as has been observed in dolomite-III and shortite under pressure (Vennari and Williams 2018; Vennari et al. 2018). The principal manifestation of this change, a softening or lowered frequency of the \({\nu }_{1}\) symmetric stretching vibration, does not occur up to 94 GPa in this material. This maintenance of the three-fold coordinate carbonate unit can likely be correlated with the maintenance of the layered-like overarching structure associated with the two lowest pressure phases: the next-closest oxygen to the carbonate units typically defines the edge of a cation polyhedron (e.g., Fig. 1).
Compressibility of compositionally and structurally diverse carbonate phases
The evident rigidity of trigonal [CO3] units in solid carbonate phases indicates that the cations play a dominant role in volumetric reduction during isothermal compression. Here, we explore the relationship between cation radius and compressibility for three groups of compositionally diverse and geologically important carbonate mineral structures: the broadly-defined “double carbonates” including bütschliite, the calcite-structured group, and the aragonite-structured group.
Figure 12 shows the relationship between the isothermal bulk modulus, \({K}_{T}\), and average non-carbon cation radius (a proxy for bond strength) for minerals belonging to each of the three carbonate groups listed above. In the case of the double carbonates, the cation radius is determined by weighting each cation radius by its stoichiometric subscript and dividing by the total number of cations (excluding carbon) in the chemical formula. It can clearly be seen that the value of the bulk modulus decreases with increasing cation radius. A linear fit to the double carbonate data yields \({K}_{T}=-94.7\left(21\right)r+175.0(20)\) and a linear fit to all data points yields \({K}_{T}=-82.4\left(11\right)r+161.3(12)\). While the separate structures and stoichiometries present in Fig. 12 may each have modestly different trends (with the trend of the transition metal-bearing calcite structured minerals having been noted previously by Sawchuk et al. 2021), the overarching trends are roughly comparable. Figure 12 thus illustrates a rather simple effect: the compaction of the carbonates is largely modulated by their charge-balancing cations. Deviations from this general trend (such as might be the case for bütschliite-II) may reflect the presence of more complex compressional mechanisms beyond simple compaction of the cation sites (such as angle changes, carbonate group rotations, and/or coordination changes).
Isothermal bulk moduli as a function of cation radius for a suite of structurally and compositionally diverse carbonate minerals. The solid black line is a fit to the double carbonates only and the dotted gray line is a fit to all data. Cation radii are taken from Shannon (1976). Bulk moduli data are from: bütschliite and bütschliite-II (K2Ca(CO3)2, this study); spherocobaltite (CoCO3, Chariton et al. 2017); eitelite (Na2Mg(CO3)2) and K2Mg(CO3)2 (Golubkova et al. 2015); siderite (FeCO3, Lavina et al. 2010); rhodochrosite (MnCO3, Merlini et al. 2015); aragonite (CaCO3, Palaich et al. 2016); calcite (CaCO3, Redfern and Angel 1999); dolomite and ankerite (CaMg(CO3)2 and Ca(Fe,Mg,Mn)(CO3)2, Ross and Reeder 1992); gaspéite ((Ni,Mg)CO3, Sawchuk et al. 2021); shortite (Na2Ca2(CO3)3, Vennari et al. 2018); strontianite and witherite (SrCO3 and BaCO3, Wang et al. 2015); cerussite (PbCO3, Yu-Feng et al. 2013); and magnesite (MgCO3, Zhang et al. 1997)
Conclusions
Bütschliite is a highly compressible mineral that undergoes a series of four high-pressure phase transitions during isothermal compression up to 95 GPa. Compression of the ambient-pressure phase is highly anisotropic, with contraction along the c-axis (i.e., reductions in K–O and K–Ca distances) accounting for most of the volume change in this material. Our results suggest that the ambient-pressure bütschliite phase is likely stable to a depth of about 180 km within the Earth; this stability range in depth is likely to be largely independent of geothermal variations.
Bütschliite converts to a monoclinic phase with C2/m symmetry at ~ 6 GPa. Compression of this phase is controlled by interlayer shifting and rotation of the carbonate groups. This new compression mechanism provides additional means of compaction that are unavailable to the low-pressure phase, and results in a more compressible structure. The enhanced compressibility of the C2/m structure likely augments the stability of the bütschliite-II phase, as the large \(P\Delta V\) difference between the \(R\overline{3 }m\) phase (in its metastable region above ~ 6 GPa) and the more compressible C2/m phase (and possibly the hypothesized \(P\overline{1 }\) phase) will result in an expanded stability field in the phase diagram. A Fermi resonance observed in the lattice modes of bütschliite-II provides independent confirmation of the relative compressibilities of different axes that were characterized with single-crystal X-ray diffraction. Our results, when kinetic effects are considered, indicate that bütschliite inclusions found in deep-formed diamonds (e.g., Logvinova et al. 2019) likely initially crystallized as the C2/m phase and reverted to the R \(\overline{3 }\) m phase during ascent through the mantle or following emplacement. Furthermore, our results also indicate that previous phase equilibrium experiments in alkali carbonate systems (e.g., Shatskiy et al. 2015) may not have crystallized bütschliite at 6 GPa, but rather the C2/m phase, which reverts to bütschliite upon quenching.
Subsequent phase transitions observed in the Raman data show progressive distortion of carbonate units, ultimately giving rise to new distinct carbonate units within the structure and possibly expansion of the unit cell. Nevertheless, the overarching layer-type structure appears to be maintained at 300 K across these transitions. The stability of bütschliite-V to very high pressures suggests that this phase could, if stable at high temperatures, play an important role in deep Earth alkaline carbonate storage and magmatism.
References
Abersteiner A, Golovin A, Chayka I, Kaminetsky V, Goemann K, Rodemann T, Ehrig K (2022) Carbon compounds in the West Kimberley lamproites (Australia): Insights from melt and fluid inclusions. Gondwana Res 109:536–557. https://doi.org/10.1016/j.gr.2022.06.005
Akahama Y, Kawamura H (2006) Pressure calibration of diamond anvil Raman gauge to 310 GPa. J Appl Phys 100:043516. https://doi.org/10.1063/1.2335683
Akella A, Keszler DA (1995a) Crystal structure of the borate Ba2Ca(BO3)2. Main Group Met Chem 18:35–42. https://doi.org/10.1515/mgmc.1995.18.1.35
Akella A, Keszler DA (1995b) Structure and Eu2+ luminescence of dibarium magnesium orthoborate. Mater Res Bull 30:105–111. https://doi.org/10.1016/0025-5408(94)00113-8
Arefiev AV, Shatskiy A, Podborodnikov IV, Litasov KD (2018) Melting and subsolidus phase relations in the system K2CO3–MgCO3 at 3 GPa. High Pressure Res 38:422–439
Arefiev AV, Shatskiy A, Podborodnikov IV, Rashchenko SV, Chanyshev AD, Litasov KD (2019) The system K2CO3–CaCO3 at 3 GPa: link between phase relations and variety of K–Ca double carbonates at ≤ 0.1 and 6 GPa. Phys Chem Miner 46:229–244
Bayarjargal L, Fruhner C-J, Schrodt N, Winkler B (2018) CaCO3 phase diagram studied with Raman spectroscopy at pressures up to 50 GPa and high temperatures and DFT modeling. Phys Earth Planet in 281:31–45. https://doi.org/10.1016/j.pepi.2018.05.002
Bermanec V, Scholz R, Markovic F, Gobac Ž, Chaves M (2011) Mineralogy of the Boa Vista pegmatite, Galileia, Minas Gerais, Brazil. In: 5th International Symposium on Granitic Pegmatites, Mendoza, Argentina, pp 33–35
Birch F (1978) Finite strain isotherm and velocities for single-crystal and polycrystalline NaCl at high pressures and 300°K. J Geophys Res 83:1257. https://doi.org/10.1029/JB083iB03p01257
Brey GP, Bulatov VK, Girnis AV (2011) Melting of K-rich carbonated peridotite at 6–10 GPa and the stability of K-phases in the upper mantle. Chem Geol 281:333–342. https://doi.org/10.1016/j.chemgeo.2010.12.019
Bruker (2016) APEX3. Bruker AXS Inc, Madison
Caracas R, Bobocioiu E (2011) The WURM project–a freely available web-based repository of computed physical data for minerals. Am Mineral 96:437–443. https://doi.org/10.2138/am.2011.3532
Chariton S, Cerantola V, Ismailova L, Bykova E, Bykov M, Kupenko I, McCammon C, Dubrovinsky L (2017) The high-pressure behavior of spherocobaltite (CoCO3): a single crystal Raman spectroscopy and XRD study. Phys Chem Miner. https://doi.org/10.1007/s00269-017-0902-5
Chayka IF, Kamenetsky VS, Vladykin NV, Kontonikas-Charos A, Prokopyev IR, Stepanov SY, Krasheninnikov SP (2021) Origin of alkali-rich volcanic and alkali-poor intrusive carbonatites from a common parental magma. Sci Rep. https://doi.org/10.1038/s41598-021-97014-y
Decius JC (1955) Coupling of the out-of-plane bending mode in nitrates and carbonates of the aragonite structure. J Chem Phys 23:1290–1294. https://doi.org/10.1063/1.1742260
Efthimiopoulos I, Müller J, Winkler B, Ozten C, Harms M, Schade U, Koch-Müller M (2019) Vibrational response of strontianite at high pressures and high temperatures and construction of P-T phase diagram. Phys Chem Miner 46:27–35. https://doi.org/10.1007/s00269-018-0984-8
Gillet P, Biellmann C, Reynard B, McMillan P (1993) Raman spectroscopic studies of carbonates part I: High-pressure and high-temperature behaviour of calcite, magnesite, dolomite and aragonite. Phys Chem Miner 20:1–18. https://doi.org/10.1007/BF00202245
Golubkova A, Merlini M, Schmidt MW (2015) Crystal structure, high-pressure, and high-temperature behavior of carbonates in the K2Mg(CO3)2–Na2Mg(CO3)2 join. Am Mineral 100:2458–2467
Harlow GE, Davies R (2004) Status report on stability of K-rich phases at mantle conditions. Lithos 77:647–653. https://doi.org/10.1016/j.lithos.2004.04.010
Hou B, Huang S, Qin S (2022) Phase transitions and compressibility of alkali-bearing double carbonates at high pressures: a first-principles calculations study. Phys Chem Miner 49:34. https://doi.org/10.1007/s00269-022-01210-9
Jeanloz R (1981) Finite-strain equation of state for high-pressure phases. Geophys Res Lett 8:1219–1222. https://doi.org/10.1029/GL008i012p01219
Kraft S, Knittle E, Williams Q (1991) Carbonate stability in the Earth’s mantle: a vibrational spectroscopic study of aragonite and dolomite at high pressures and temperatures. J Geophys Res 96:17997. https://doi.org/10.1029/91JB01749
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169–11186. https://doi.org/10.1103/PhysRevB.54.11169
Kunz M, Brown ID (1995) Out-of-center distortions around octahedrally coordinated d0 transition metals. J Sol State Chem 115:395–406
Lavina B, Dera P, Down RT, Yang W, Sinogeikin S, Meng Y, Shen G, Schiferl D (2010) Structure of siderite FeCO3 to 56 GPa and hysteresis of its spin-pairing transition. Phys Rev B 82:0641110
Logvinova AM, Shatskiy A, Wirth R, Tomilenko AA, Ugap’eva SS, Sobolev NV (2019) Carbonatite melt in type Ia gem diamond. Lithos 342–343:463–467
Lv X, Wei L, Wang X, Xu J, Yu H, Hu Y, Zhang H, Zhang C, Wang J, Li Q (2018) Crystal growth, electronic structure and optical properties of Sr2Mg(BO3)2. J Solid State Chem 258:283–288. https://doi.org/10.1016/j.jssc.2017.10.032
McKie D (1990) Subsolidus phase relations in the system K2Ca(CO3)2–Na2Mg(CO3)2 at 1 kbar; the fairchilditess-buetschliite-eitelite eutectoid. Am Mineral 75:1147–1150
Meltzer A, Kessel R (2023) The metasomatism of sub-cratonic peridotites by a slab-derived fluid—implications for cratons’ evolution. Geochim Cosmochim Acta 350:28–45. https://doi.org/10.1016/j.gca.2023.03.034
Merlini M, Hanfland M, Gemmi M (2015) The MnCO3-II high-pressure polymorph of rhodochrosite. Am Mineral 100:2625–2629
Milton C, Axelrod J (1947) Fused wood-ash stones: fairchildite (n. sp.) K2CO3·CaCO3, buetschliite (n. sp.) 3K2CO3·2CaCO3·6H2O and calcite, CaCO3, their essential components. Am Mineral 32:607–624
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Cryst 44:1272–1276. https://doi.org/10.1107/S0021889811038970
Mrose ME, Rose HJ, Marinenko JW (1966) Synthesis and properties of fairchildite and buetschliite: their relation to wood-ash stone formation. In: Program of the Geol Soc. Am. Meeting, San Francisco
Pabst A (1974) Synthesis, properties, and structure of K2Ca(CO3)2, buetschliite. Am Mineral 59:6
Palaich SE, Heffern RA, Hanfland M, Lausi A, Kavner A, Manning CE, Merlini M (2016) High-pressure compressibility and thermal expansion of aragonite. Am Mineral 101:1651–1658
Peterson VK (2005) Lattice parameter measurement using Le Bail versus structural (Rietveld) refinement: a caution for complex, low symmetry systems. Powder Diffr 20:14–17. https://doi.org/10.1154/1.1810156
Rashchenko SV, Goryainov SV, Romanenko AV, Golovin AV, Korsakov AV, Bn M, Mikhno AO (2017) High-pressure Raman study of nyerereite from Oldoinyo Lengai. J Raman Spectrosc 48:1438–1442. https://doi.org/10.1002/jrs.5152
Redfern SA, Angel RJ (1999) High-pressure behaviour and equation of state of calcite, CaCO3. Contrib Mineral Petr 134:102–106
Ross NL, Reeder RJ (1992) High-pressure structural study of dolomite and ankerite. Am Mineral 77:412–421
Santamaria-Perez D, Pavic L, Chuliá-Jordán R, Ruiz-Fuertes J, Popescu C, Otero-de-la-Roza A (2023) Phase stability of stress-sensitive Ag2CO3 silver carbonate at high pressures and temperatures. Solid State Sci 135:107068. https://doi.org/10.1016/j.solidstatesciences.2022.107068
Sawchuk K, Kamat R, McGuire C, Kavner A (2021) An X-ray diffraction and Raman spectroscopic study of the high-pressure behavior of gaspéite (Ni0.73Mg0.27CO3). Phys Chem Miner 48:7. https://doi.org/10.1007/s00269-020-01133-3
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A 32:751–767. https://doi.org/10.1107/S0567739476001551
Shatskiy A, Borzdov YM, Litasov KD, Sharygin IS, Palyanov YN, Ohtani E (2015) Phase relationships in the system K2CO3-CaCO3 at 6 GPa and 900–1450°C. Am Mineral 100:223–232. https://doi.org/10.2138/am-2015-5001
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A 64:112–122
Shen G, Wang Y, Dewaele A, Wu C, Fratanduono DE, Eggert J, Klotz S, Dziubek KF, Loubeyre P, Fat’yanov OV, Asimow PD, Mashimo T, Wentzcovitch RMM et al (2020) Toward an international practical pressure scale: a proposal for an IPPS ruby gauge (IPPS-Ruby2020). High Pressure Res 40:299–314
Shimizu H (1985) Pressure-tuning resonance between the vibron and the libron in CH2Br2 and CD2Br2 molecular solids. Phys Rev B 32:4120–4125
Tian H, Li Y, Zhang Q, Ningning S, Sun J, Xiao N, Liu Y, Zhang J, Liu H, Li Y (2023) Pressure-dependent compressibility of K2X(CO3)2 (X = Ca, Mg) under high pressure. Mater Lett 349:134895. https://doi.org/10.1016/j.matlet.2023.134895
Toby BH, Von Dreele RB (2013) GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J Appl Cryst 46:544–549. https://doi.org/10.1107/S0021889813003531
Vennari CE, Williams Q (2018) A novel carbon bonding environment in deep mantle high-pressure dolomite. Am Mineral 103:171–174
Vennari CE, Beavers CM, Williams Q (2018) High-pressure/temperature behavior of the alkali/calcium carbonate shortite (Na2Ca2(CO3)3): implications for carbon sequestration in Earth’s transition zone. J Geophys Res Solid Earth 123:6574–6591
Vennari CE, Williams Q (2019) High-pressure Raman and Nd3+ luminescence spectroscopy of bastnäsite–(REE)CO3F. Am Mineral 104:1389–1401. https://doi.org/10.2138/am-2019-7011
Wang M, Liu Q, Nie S, Li B, Wu Y, Gao J, Wei X, Wu X (2015) High-pressure phase transitions and compressibilities of aragonite-structure carbonates: SrCO3 and BaCO3. Phys Chem Miner 42:517–527. https://doi.org/10.1007/s00269-015-0740-2
Wood RM, Palenik GJ (1999) Bond valence sums in coordination chemistry using new R0 Values. Potassium-oxygen complexes. Inorg Chem 38:1031–1034
Yu-Feng Z, Jing L, Zhen-Xing Q, Chuan-Long L, Lun X, Rui L, LiGang B (2013) A high-pressure study of PbCO3 by XRD and Raman spectroscopy. Chin Phys C 37(3):038001. https://doi.org/10.1088/1674-1137/37/3/038001
Zhang J, Martinez I, Guyot F, Gillet P, Saxena SK (1997) X-ray diffraction study of magnesite at high pressure and high temperature. Phys Chem Miner 24:122–130. https://doi.org/10.1007/s002690050025
Zhuravlev YuN (2022) Vibration spectra of double K-Ca, K–Mg, and Na–Mg carbonates under pressure. Geochem Int 60:1103–1113. https://doi.org/10.1134/S0016702922110118
Acknowledgements
This work was supported by the US National Science Foundation through award EAR-2017294. This research used resources of the Advanced Light Source (beamline 12.2.2) at Lawrence Berkeley National Laboratory, which is DOE Office of Science User facility under Contract No. DE-AC02-05CH11231. This research was partially supported by COMPRES, the Consortium for Materials Properties Research in Earth Sciences under NSF Cooperative Agreement EAR 1606856, and SEES, Synchrotron Earth and Environmental Science under NSF Cooperative Agreement EAR 2223273.
Funding
This study was supported by National Science Foundation (EAR-2017294, EAR 2223273, EAR 2223273, EAR-2017294) and U.S. Department of Energy (DE-AC02-05CH11231).
Author information
Authors and Affiliations
Contributions
GZ and QW designed the project, GZ, BK, KA, MK conducted the experiments, GZ and BK analyzed the data with input from all authors, and all authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zeff, G., Kalkan, B., Armstrong, K. et al. High pressure raman spectroscopy and X-ray diffraction of K2Ca(CO3)2 bütschliite: multiple pressure-induced phase transitions in a double carbonate. Phys Chem Minerals 51, 2 (2024). https://doi.org/10.1007/s00269-023-01262-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-023-01262-5