Abstract
Sorption of heavy metal cations (Pb(II), Cr(III), Cd(II), Ni(II)) from aqueous solutions on natural Na-clinoptilolite was studied using atomic absorption spectrometry (AAS) and FT-IR spectroscopy. It was found that the sorption capacity of clinoptilolite decreases in the following order: Pb(II) (22,600 mg/kg), Cr(III) (21,200 mg/kg), Cd(II) (10,400 mg/kg) and Ni(II) (6,200 mg/kg). In the FT-IR spectra of the samples, in the region of pseudolattice vibrations (500–800 cm−1), systematic changes connected with the type of cation and its concentration in the initial solution were observed. The proportions of ion exchange and chemisorption in the whole process of sorption were also estimated. It was found that the amount of cations sorbed on clinoptilolite depended on the concentrations and pH of the solutions used as well as on the contact time of zeolite-solution system. After 120 min of the reaction, despite the metal type, 90–100% of the total amount of cations were immobilized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among natural and synthetic microporous materials, zeolites constitute a major group with unique physicochemical properties. Due to these properties, zeolites have been widely applied as molecular sieves, ion-exchange units, adsorbents and catalysts in industry and environment protection (Breck 1974). They are also used for removal of heavy metal species from industrial waste-water (e.g. Pansini 1996; Kayabali and Kezer 1998; Ouki and Kavannagh 1999; Mier et al. 2001). Heavy metal cations can be immobilized by zeolites by two mechanisms: ion exchange and chemisorption (Jenne 1998). Ion exchange involves substitution of ions present in zeolite crystalline lattice by metal ions from the solution (Inglezakis et al. 2002). The type of cation (the position of the cation in the selectivity series) as well as the cation concentration in the solution will determine the ion-exchange efficiency. Ion-exchange properties of zeolites are due to the presence of non-compensated negative charges, which originate from heterovalence substitution (Si4+ by Al3+ in tetrahedral positions) as well as the presence of surface functional groups (mainly OH− and H2O) in crystalline lattice discontinuity points. As the result of lattice negative charge compensation by metal cation, outer-sphere or inner-sphere complexes are formed. Stability of the outer-sphere complexes is very low because the binding of the zeolite with the metal ion complexed is in such a way that intermolecular forces dominate (McBride 2000).
In contrast, the second mechanism of metal ions immobilization, i.e. chemisorption, results in the formation of stable inner-sphere complexes (Godelitsas 1999). This is due to the fact that functional groups (mainly OH−) form strong chemical bonds with metal ions outside the hydration envelope (Jenne 1998). In zeolites, ion-exchange processes generally dominate chemisorption.
The atomic absorption spectrometry (AAS) method is very often used for the quantitative analysis of heavy metal immobilization processes (e.g. Langella et al. 2000). This method enables the determination of the amount of sorbed ions with high accuracy. It can be estimated by comparison of the solution concentration before and after the process of sorption. The incorporation of new nontetrahedral cations into the zeolite structure should cause changes in the infrared (IR) spectra. These changes are expected particularly in the pseudolattice bands connected with the presence of alumino- and silicooxygen tetrahedral rings in the zeolite structure.
Determination of the capability of clinoptilolite to immoblize heavy metal cations based on the results of AAS and IR spectroscopic studies has been the main goal of the present work.
Experimental
Heavy metal cations were immobilized in a sodium form of natural Polish clinoptilolite, obtained by the procedure described in the previous work (Mozgawa 2000). The results of the analysis of initial zeolite composition (expressed as if the zeolite contained anhydrous oxides) were as follows (in wt%): SiO2—77.66, Al2O3—13.77, CaO—3.2, K2O—1.67, SrO—1.34, BaO—0.91, MgO—0.88, Fe2O3—0.37% and Na2O—0.17% (Mozgawa 2000).
Sorption of Pb(II), Cd(II), Ni(II) and Cr(III) on the zeolite was studied using aqueous solutions of metal nitrates or chlorides. Several solutions of various metal concentrations (10–70–150–400–900–1,800 mg/dm3) were used in the investigations. pH of these solutions was not modified.
In a typical sorption experiment, a suspension of the zeolite in water (20 g/dm3) was shaken with the appropriate metal salt solution for 24 h at 25°C in a rotary shaking thermostat. The suspension was then centrifuged at 10,000 rpm for 10 min. Based on the studies of the influence of time on the amount of the metals sorbed, it was found that 24 h is a sufficient period of time to reach the equilibrium state between the metal ions in the solution and sorbed on clinoptilolite (Bajda et al. 2004).
Atomic absorption spectrometry (Philips PU-9100x) was used to determine the concentration of metal cations in the solutions before and after the sorption experiments. Additionally, pH of these solutions was controlled.
To desorb the sorbed metals, the zeolite samples were flushed twice with redistilled water and flooded with 1 M ammonium acetate aqueous solution at the zeolite to solution ratio 1:50. Use of ammonium ion in desorption experiments assures removal of all metal ions from external and internal cation-exchange positions of clinoptilolite (Calmano and Förstner 1983; Ming and Dixon 1987; Li and Bowman 1997; Çulfaz and Yağiz 2004). In order to remove 60 metal ions sorbed in the ion-exchange positions of the zeolite, a repeated ion-exchange procedure is usually necessary. However, according to Calmano and Förstner (1983) if the concentration of NH4 ion in desorbing solution is 1 M and the solid phase:solution ratio is at least 1:20 then a single desorption experiment guarantees that the equilibrium amount of the desorbed ion is reached.
After 2 h of shaking, the samples were centrifuged, as described above. Metal ion concentrations in the obtained solutions were determined using AAS.
To study the influence of contact time with respect to the amount of Pb(II), Cd(II), Ni(II) and Cr(III) sorbed, the zeolite samples were shaken with the solutions of constant metal concentration (900 mg/dm3). After each period of time (1–2–5–10–20–40–60–120–240–600 min), pH and metal concentration were measured. All samples were triplicated.
Infrared spectra were measured on a Bio-Rad FTS-60 spectrometer. Spectra were collected after 256 scans at 2 cm−1 resolution in the region of 4,000–400 cm−1. Samples were prepared using the standard KBr pellets method.
Results and discussion
Results of heavy metal immobilization processes as the function of the equilibrium metal ion concentration in the solution are presented in Figs. 1 and 2. In the diagrams, the curves representing the total amount of metal immobilized on the zeolite (denoted as sorption), together with the curves of chemisorption, ion exchange and pH variation in the solution after sorption, are shown. Chemisorption levels were determined based on the desorption experiments. In the desorption process, heavy metal cations incorporated into the zeolite via ion exchange were substituted by NH +4 cations from ammonium acetate, whereas, those immobilized via chemisorption remained in the structure.
It has been found that effects of chemisorption and ion exchange on the overall immobilization of metal cations in clinoptilolite depends on the type of metal. The chemisorption process influences immobilization of lead and particularly chromium to a high extent (Fig. 1). In the case of chromium, the contribution from ion exchange is insignificant, which confirms that for the trivalent cations the ion exchange in clinoptilolite proceeds to a small extent. In the case of lead, the chemisorption and ion exchange become equally important at higher metal ion concentration in the initial solution. In the case of cadmium, the chemisorption processes slightly predominate, whereas in the case of nickel ion-exchange processes prevail (Fig. 2).
At the highest initial concentration of ions of all metals in the solutions, a small decrease in the chemisorption contribution can be observed. This can be related to the pH changes. Despite pH decrease in the concentration range up to about 1000 mg/dm3, the amount of sorbed ions increases. It should be noted, however, that after reaching the saturation level of a metal ion sorbed, further pH lowering causes the increase in the zeolite crystalline lattice positive charge, which reduces the zeolite ability of metal cations chemisorption. The participation of ion-exchange process, which is influenced by pH changes to a smaller extent, still continues. This phenomenon is especially distinct at larger pH difference, i.e. for two solutions of the highest initial metal ions concentrations (Figs. 1, 2).
The proportion of chemisorption and ion-exchange processes depends on inherent properties of the metal. Thus, in the case of cadmium and lead, chemisorption predominates; whereas in the case of nickel ion exchange prevails (Figs. 1, 2). For all the metals, a decrease in the share of chemisorption at the highest equilibrium concentration is observed. This phenomenon is caused by the change in the pH of the equilibrium solution. At lower starting concentrations (up to 480–1,000 mg/dm3), the amount of the sorbed metal grows in spite of pH lowering. (Figs. 1, 2). At higher concentrations of the metal in the solution, the sorbed amount does not grow since the saturation of the clinoptilolite sorption complex with a given metal is reached. On the other hand, increase in the concentration of the starting solution is accompanied by further pH lowering. This results in the growth of the zeolite crystalline lattice positive charge and, as a consequence, relative lowering of the cation chemisorption contribution in the immoblization process. The higher the difference in the last analytical points (the highest equilibrium metal concentrations in the solutions) the more pronounced the phenomenon (Fig. 1—Pb, Fig. 2—Ni). pH lowering is caused by the increase in H+ ions concentration in the solution. Since H+ are less competitive with respect to the metal ions studied in the ion-exchange process, the share of ion exchange increases or stays constant in spite of pH lowering.
The total amount of heavy metal ions sorbed on clinoptilolite is a measure of its sorption capacity. Of all the metals studied, sorption capacity decreases in the following order: Pb (22,600 mg/kg), Cr (21,200 mg/kg), Cd (10,400 mg/kg) and Ni (6,200 mg/kg).
The AAS results have been then compared with IR spectroscopic studies. The essential changes in IR spectra caused by heavy metals sorption are observed in the range of the pseudolattice vibrations, i.e. 700–500 cm−1 as it has been shown in the previous study (Mozgawa 2000), whereas the remaining bands in the IR spectra are almost unchanged. The bands in the range of 705–660 cm−1 due to the vibrations of 4- and 6-membered rings in the zeolite structure, have been analysed in more detail. In this range, the bands at about 693 and 674 cm−1 occur. The latter band is sensitive to changes in the type and the amount of non-tetrahedral cations. This band is connected with symmetrical stretching vibrations of Si–O bond existing in the 6-membered rings of the zeolite (Flanigen et al. 1971). The change of integral intensity of this band can give information on the amount of heavy metal cations sorbed in the zeolite structure. In Fig. 3, the IR spectra of clinoptilolite after sorption of cadmium (from the solutions of various initial concentrations), in the range of 705–660 cm−1, are presented. In this spectra, an increase in the intensity of the 674 cm−1 band with the increase in the initial concentration of the solution, can be observed. Precise estimation of intensity changes is possible after decomposition of the spectra into component bands. Spectra decomposition has been carried out following the method proposed by Handke et al. (1994), using Win-IR programme. For all the spectra, the baseline correction has been carried out before the decomposition process. The example of such decomposition for three different initial solution concentrations is also shown in Fig. 3. The bands intensity is expressed as the participation of the integral intensity of each band in the sum of the intensity of both bands (in percentage). The intensity of the “analytical” band for all concentrations is shown in Table 1. Systematic increase in the intensity of the 674 cm−1 band (with respect to the 693 cm−1 band) with increasing solution concentration is noticeable. However, the observed changes are much smaller and show a different course in comparison with the AAS results. Similar observations are valid in the case of Pb, Cr and Ni sorption (Figs. 3, 4, Table 1). Thus, the band at 674 cm−1 can be considered as an “indicator” whose intensity changes after heavy metal sorption. However, to estimate precisely the amount of ions sorbed, another analytical method should be used (e.g. AAS).
The change in the 674 cm−1 band intensity should be related to the non-tetrahedral cations exchange (the exchange of Na+ cations with heavy metal cations). This process causes the change in the ring environment related to the change of ion charge, ion radius and ring deformation. This influences the intensity of ring vibrational bands. It can be proposed that ion exchange mainly causes the changes of the spectra in the pseudolattice region. This is clearly visible in the case of chromium (Fig. 4), which shows insignificant contribution to ion exchange (as it has been determined on the basis of the AAS results).
In Figs. 5 and 6, the results of experimental metals sorption on clinoptilolite as the function of time are shown. It can be seen that immobilization of Cr is the fastest process, followed by the immobilization of Pb, Cd, and Ni. However, even though the amount of Cr sorbed is lower than that of Pb, sorption of Cr is a little faster in the first two minutes of the process (with respect to the whole amount of metal cation sorbed).
Sorption values presented in figures are expressed in milligram of metal/kilogram of the sorbent. In order to compare the kinetics of sorption of various metals, these values have been transformed into moles. It has been found that after this procedure the sequence of sorption rate has not changed.
After the first two minutes of the reaction, 76% of the total amount of Cr was immobilized. In the case of other metals, these amounts were as follows: 71% Pb, 64% Cd and 49% Ni. After 120 min of the reaction, 90–100% of metal ions were immobilized regardless of the metal type. The contribution of chemisorption and ion-exchange processes to the metal immobilization on clinoptilolite depends on the metal type and the reaction time. In the case of lead, after the first minutes of reaction, chemisorption becomes more dominant than the ion-exchange process (Fig. 5). Chromium is immobilized by chemisorption independently of the reaction time (Fig. 5). In the case of reaction between the zeolite and cadmium, almost all ion-exchange positions are filled within the first few minutes. The share of chemisorption increases but it does not reach the ion-exchange level. Over two times more Cd(II) is chemisorbed than ion-exchanged. In the case of nickel, the shares of chemisorption and ion exchange are comparable within the first minutes of the reaction. After this period of time, the effect of both processes become equal. After 120 min, chemisorption slightly predominates. Of all the metal cations studied, the ion-exchange process is the fastest. Ion-exchange equilibrium is reached within the first minutes of the reaction. Further increase in the reaction time does not lead to the growth of the amount of ion-exchanged metals. This is due to the lower binding energy of cations in the ion-exchange sites and better accessibility of these sites than the active centres responsible for the chemisorption processes. Proportions of the amounts of metal firmly sorbed and the ion exchange depend mainly on the nature of the cation: its oxidation level, ion radius, solvation energy, concentration of the solution from which it is sorbed and the speciation which it forms in this solution as well as the type of the material on which the metal is sorbed. In the systems studied, the proportion of chemisorption and ion exchange lowers in the following order: Cr(III) > Pb(II) > Ni(II) > Cd(II). This order is caused by both the inherent properties of the metals and the mechanism of their binding with clinoptilolite.
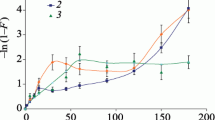
Kinetics of sorption refers to the rate of reaching the equilibrium state between the metal bound to the zeolite and the metal concentration in the surrounding solution. Theoretical models of sorption kinetics have been presented in the paper by Ho and McKay (1999). The line obtained after plotting log(qeq −q t ) versus t, and t/q t versus t shows the degree of fitting of the metal sorption to the pseudo-first and pseudo-second order rate kinetics model, respectively (Fig. 7). Most frequently, kinetics of sorption of metal cations on clinoptilolite is described using the pseudo-first or pseudo-second order models (Ho and McKay 1999). Therefore in our work, these two models have been compared. Kinetic data of Pb(II), Cr(III), Cd(II) and Ni(II) sorption can be more appropriately defined by the pseudo-second order sorption rate than the pseudo-first order one (Fig. 7) because R2 (correlation coefficient) values are higher for the pseudo-second order compared to the pseudo-first order sorption rate.
In Figs. 8 and 9, a set of the IR clinoptilolite spectra in the range of 705–660 cm−1, after different sorption time for various cations are shown. It is clearly seen that the intensity of the band at 674 cm−1 increases distinctly in the spectra measured after 1, 2, 5 or 10 min of sorption. These changes are not so sharp after further increase in the reaction time. This confirms that the sorption of heavy metal cations on clinoptilolite is a fast process. The spectra obtained for the samples after different sorption times were decomposed in similar manner as those corresponding to the sorption from the solutions of various initial concentrations. The results are presented in Table 2. When the results for different cations are compared, it can be observed that the smallest changes occur in the IR spectra after Cr sorption (Fig. 8). The intensity of the band at 674 cm−1 changes from 51% after 1 min to 69% after 10 h but when these spectra are carefully examined, no distinct differences can be seen. A similar tendency can be observed in the case of Pb. In contrast, sorption of Cd seems to increase after 5 min because the band at 674 cm−1 shows markedly higher intensity in the spectrum after 10 min of sorption. The sorption process of nickel increases significantly after 1 min. The obtained results are comparable with AAS results.
Conclusions
-
1.
The cationic forms of Pb(II), Cd(II), Cr(III) and Ni(II) are immobilized in clinoptilolite structure by two mechanisms: ion exchange and chemisorption. In the case of lead and chromium, chemisorption predominates. The participation of both mechanisms in the case of cadmium and nickel seems to be equal.
-
2.
Incorporation of heavy metal cations causes changes in the IR spectra in the range of 705–660 cm−1. The increase in the initial concentration of the solutions from which cations are introduced as well as the reaction time causes the increase in the integral intensity of the band at 674 cm−1. The trends of these changes, however, are quite different from the results obtained using AAS method.
-
3.
Changes of the IR spectra in the pseudo-lattice region are connected mainly with the ion-exchange process (the exchange of non-tetrahedral cations). Chemisorption influences this process to a less degree.
-
4.
In the process of immobilization of chromium, ion exchange contributes to a low extent. Almost total amount of immobilized metal ions is incorporated into the zeolite structure by chemisorption.
-
5.
Clinoptilolite sorption capacity was determined. Among the metals studied, Pb was incorporated in the highest amount 22,600 mg/kg. The amount of sorbed chromium is equal to 21,200 mg/kg, while in the case of cadmium and nickel it is 10,400 mg/kg and 6,200 mg/kg, respectively.
-
6.
The process of sorption of heavy metal cations on clinoptilolite is relatively fast. After 120 min of the reaction, despite the metal type, 90–100% of the total amount of cations were immobilized. Sorption of Cr on zeolites is the fastest, while Pb, Cd and Ni are sorbed more slowly.
References
Bajda T, Franus W, Manecki A, Manecki M, Mozgawa W, Sikora M (2004) Sorption of heavy metals on natural zeolite and smectite–zeolite shale from the Polish Flysch Carpathians. Pol J Environ Stud 13(Suppl III):7–10
Breck DW (1974) Zeolite Molecular Sieves. Wiley, NY, London, Sydney, Toronto
Calmano W, Förstner U (1983) Chemical extraction of heavy metals in polluted river sediments in central Europe. Sci Total Environ 28:77–90
Çulfaz M, Yağiz M (2004) Ion-exchange properties of natural clinoptilolite: lead–sodium and cadmium–sodium equilibria. Sep Purif Technol 37:93–105
Godelitsas A (1999) Transition metal complexes supported on natural zeolitic materials: an overwiew. In: Misaelides P et al (eds) Natural microporous materials in environmental technology. Kluwer, Dordrecht, pp 271–281
Handke M, Mozgawa W, Nocuń M (1994) Specific features of IR spectra of silicate glasses. J Mol Struct 325:129–136
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Proc Biochem 34:451–465
Flanigen EM, Khatami H, Szymanski HA (1971) Infrared structural studies of zeolite frameworks. Adv Chem Ser 101:201–229
Inglezakis VJ, Loizidou MD, Grigoropoulou HP (2002) Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res 36:2784–2792
Jenne EA (1998) Adsorption models. In: Jenne EA (ed) Adsorption of metals by geomedia: variables, mechanism and model applications. Academic, San Diego, pp 11–36
Kayabali K, Kezer H, (1998) Testing the ability of bentonite-amended natural zeolite (clinoptilolite) to remove heavy metals from liquid waste. Environ Geol 34:95–102
Langella A, Pansini M, Cappelletti P, de’Gennaro B, de’Gennaro M, Colella C (2000) NH +4 , Cu2+, Zn2+, Cd2+ and Pb2+ exchange for Na+ in a sedimentary clinoptilolite, North Sardinia, Italy. Micropor Mesopor Mater 37:337–343
Li Z, Bowman RS (1997) Counterion effects of the sorption of cationic surfactant and chromate on natural clinoptilolite. Environ Sci Technol 31:2407–2412
McBride MB (2000) Chemisorption and precipitation reactions. In: M.E. Sumner (ed) Handbook of soil science. CRC Press, New York, pp B265–B302
Mier MV, Callejas RL, Gehr R, Cisneros BEJ, Alvarez PJJ (2001) Heavy metal removal with mexican clinoptilolite: multi-component ionic exchange. Water Res 35:373–378
Ming DW, Dixon JB (1987) Quantitative determination of clinoptilolite in soils by a cation-exchange capacity method. Clays Clay Miner 35:463–468
Mozgawa W (2000) The influence of some heavy metal cations on the FTIR spectra of zeolites. J Mol Struct 555:299–304
Ouki SK, Kavannagh M (1999) Treatment of metals-contaminated wastewaters by use of natural zeolites. Water Sci Techol 39:115–122
Pansini M (1996) Natural zeolites as cation exchangers for environmental protection. Mineral Deposita 31:563–575
Acknowledgements
This work was supported by the State Committee for Scientific Research (KBN) under grant no 3 T08D 039 26.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mozgawa, W., Bajda, T. Spectroscopic study of heavy metals sorption on clinoptilolite. Phys Chem Minerals 31, 706–713 (2005). https://doi.org/10.1007/s00269-004-0433-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-004-0433-8