Abstract
Background
Up to 25% of patients with acute pancreatitis develop severe complications and are classified as severe pancreatitis with a high death rate. To improve outcomes, patients may require interventional measures including surgical procedures. Multidisciplinary approach and best practice guidelines are important to decrease mortality.
Methods
We have conducted a retrospective analysis from a prospectively maintained database in a low-volume hospital. A total of 1075 patients were attended for acute pancreatitis over a ten-year period. We have analysed 44 patients meeting the criteria for severe acute pancreatitis and for intensive care unit (ICU) admittance. Demographics and clinical data were analysed. Patients were treated according to international guidelines and a multidisciplinary flowchart for acute pancreatitis and a step-up approach for pancreatic necrosis.
Results
Forty-four patients were admitted to the ICU due to severe acute pancreatitis. Twenty-five patients needed percutaneous drainage of peri-pancreatic or abdominal fluid collections or cholecystitis. Eight patients underwent endoscopic retrograde cholangiopancreatography for choledocholithiasis and biliary sepsis or pancreatic leakage, and one patient received endoscopic trans-gastric endoscopic prosthesis for pancreatic necrosis. Sixteen patients underwent surgery: six patients for septic abdomen, four patients for pancreatic necrosis and two patients due to abdominal compartment syndrome. Four patients had a combination of surgical procedures for pancreatic necrosis and for abdominal compartment syndrome. Overall mortality was 9.1%.
Conclusion
Severe acute pancreatitis represents a complex pathology that requires a multidisciplinary approach. Establishing best practice treatments and evidence-based guidelines for severe acute pancreatitis may improve outcomes in low-volume hospitals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
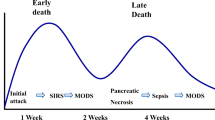
Acute pancreatitis (AP) is one of the most frequent reasons for hospital admittance due to biliopancreatic diseases [1, 2]. The main aetiologies of AP are biliary in origin or related to alcohol consumption, although in other cases, the origin of the disease is unknown [3]. Most patients have an uneventful course, but up to 25% of all patients with AP develop severe complications and are classified as having severe acute pancreatitis (SAP) with an associated death rate of up to 30–50% [2,3,4]. The incidence of AP in Spain is approximately 35–40 cases/100,000 population yearly, and its epidemiology is comparable to that in other European countries [2]. The treatment of AP and, in particular, SAP has progressively evolved and improved. One of the reasons for this improvement is that step-by-step algorithms have been incorporated into the decision-making process [5, 6]. Similarly, a step-up approach has been proposed for pancreatic necrosis [7]. To offer treatment in accordance with the severity of the disease, we need to obtain an accurate view of the expected evolution and prognosis. For that purpose, patients must be stratified based on standard classification systems. One of the first classification systems is the Ranson criteria, which use several types of laboratory and clinical data to predict mortality [8, 9]. Radiological features observed on CT scans that suggest pancreatic necrosis and the appearance of the pancreas are also used to predict mortality [10]. Simplified Acute Physiology Score version 3 (SAPS 3) is a mathematical model that predicts mortality and is widely used in intensive care units (ICUs). It is a useful index for severely ill patients [11]. The revised Atlanta classification of acute pancreatitis lists two phases of the disease, early and late, and classifies severity as mild, moderate and severe based on clinical and radiological features [5, 12]. The Sequential Organ Failure Assessment (SOFA) score is used to describe organ dysfunction/failure and is a helpful tool for evaluating the need for ICU admission [13]. In addition to medical treatment for single- or multiple-organ failure, various complications may require percutaneous, endoscopic or surgical procedures. Surgery is required for abdominal sepsis due to hollow viscera necrosis/perforation or persistent abdominal sepsis despite medical treatment and percutaneous procedures. Abdominal compartment syndrome (ACS) is associated with high complication and mortality rates. The failure of aggressive medical and non-operative treatments is expected to lead to surgery after multidisciplinary evaluation [6, 14, 15]. Necrotizing pancreatitis with infected pancreatic necrosis is associated with high mortality rates. The step-up approach to pancreatic necrosis may be completed via retroperitoneal access to solve the pancreatic focus of sepsis [6, 7]. However, early open access to pancreatic necrosis has also been advocated [16]. SAP is a challenging disease and is associated with high mortality and morbidity rates as well as high costs related to its treatment; therefore, it has been suggested that patients should be transferred to referral centres. The International Association of Pancreatology (IAP) and the American Pancreatic Association (APA) guidelines recommend transferring patients with SAP and those who may need interventional radiological, endoscopic or surgical procedures to a specialist centre. This statement is based on a strong agreement but low-quality evidence. A specialist centre for the management of AP is defined as a high-volume centre with up-to-date intensive care facilities including different treatment options. This recommendation is based on weak agreement and low-quality evidence, as there are no studies comparing the requirements for specialist centres [6]. High-volume hospitals that treat AP have been defined based on a nationwide US study that supports the criterion of more than 118 AP patients per year [17]. A nationwide Japanese study found better outcomes and shorter lengths of stay for patients with moderate and severe pancreatitis in high-volume hospitals than in low-volume hospitals, but there is still a lack of accuracy in terms of the volume of admitted patients [18]. We suggest that the implementation of best practice guidelines and multidisciplinary protocols may also be important factors in terms of outcomes. The aim of this study was to investigate the results of treatment in patients with SAP in a low-volume hospital with a multidisciplinary approach and a step-by-step comprehensive flowchart design.
Materials and methods
This study is the result of a retrospective analysis of data from a prospectively maintained database. The main objective was to evaluate the results in a single low-volume hospital in which a multidisciplinary approach, best practice guidelines and updated protocols have been applied for SAP patients from 2008 to 2018. Our hospital is a public institution that is included in the Madrid (Spain) public healthcare system with 250 beds and 12 ICU rooms. Within this time frame, 1075 patients with a diagnosis of AP were treated at our hospital. Out of this group, 44 patients were admitted to the ICU due to SAP. The total number of admissions to the ICU was 52, as some of the patients presented during the period of study with more than one episode of SAP. We used the SAPS 3 severity index to predict mortality. Severe acute pancreatitis was defined according to the modified Atlanta Classification [5], with consideration given to the Ranson and the computed tomography severity index (CTSI) [9, 10]. Patients with SAP and a SOFA score indicating organ dysfunction/failure were admitted to the ICU [19]. Based on their local and general conditions, the following patients were admitted to the ICU: all patients with SAP and high CTS index values (local condition) and patients with SAP and SOFA scores >2 for at least one organ (general condition). We also agreed to consider ICU admittance for continuous monitoring to detect clinical deterioration in patients with SAP and low and medium CTS index values and SOFA scores >1 for at least one organ (local and general conditions). Organ failure was defined as a SOFA score >2 for each parameter. Whenever a patient was diagnosed with ACS, an aggressive non-surgical strategy was adopted. In the circumstance of the failure of non-surgical treatment, a decompressive laparotomy and temporary abdominal wall closure was performed until improvement of the patient’s condition. Upon improvement of the general and local conditions of the patient, definitive abdominal wall closure was performed. Otherwise, abdominal wall repair was postponed and performed later. Descriptive data from our group of patients are shown in Table 1. A multidisciplinary flowchart that includes ICU resources, gastroenterologists, radiologists and general surgeons was initiated, and a daily decision-making process was activated (Fig. 1). Early detection of severity on ward was also incorporated after the ICU stay [20, 21].
Statistical analysis
Quantitative variables are expressed as median and interquartile ranges due to the small sample size. Qualitative variables are described as absolute numbers and percentages.
Results
All patients meeting the required criteria were admitted to the ICU. Haemodynamic and respiratory support as well as renal replacement therapy was administered whenever needed. Treatment procedures are summarized in Table 2. Twenty-five patients presented renal failure, and nine of them required renal replacement therapy. Twenty-five patients underwent percutaneous drainage of abdominal or retroperitoneal fluid collections. Two patients required percutaneous cholecystostomy for acute cholecystitis. In 17 patients, a single drainage was sufficient, while 8 patients required 2 or more drainage procedures or needed drainage tube replacement. Eight patients underwent endoscopic retrograde cholangiopancreatography (ERCP). Among them, three patients underwent urgent ERCP due to common bile duct obstruction and cholangitis less than 7 days after the onset of SAP. Two patients received ERCP for pancreatic fistula associated with peri-pancreatic fluid collections and massive pleural effusion one month after the onset of SAP, and one patient received ERCP for pancreatic duct disruption. Two patients underwent ERCP for peri-pancreatic fluid collections that were not accessible percutaneously, and one patient underwent sustained pancreatic leakage after retroperitoneoscopy and pancreatic necrosectomy. One patient underwent trans-gastric retroperitoneal drainage with endoscopic prosthesis. Patients were conservatively treated unless deterioration occurred due to a septic abdomen (six patients); there was a failure to resolve pancreatic necrosis by other means (eight patients) or ACS was identified (six patients). In those cases, an urgent laparotomy was performed. Four patients underwent right colectomy due to right colon necrosis, and one patient experienced duodenal perforation after ERCP. One patient underwent surgery due to biliary peritonitis caused by gallbladder perforated cholecystitis. In six patients, a bilateral subcostal laparotomy with a mesh-mediated temporary abdominal closure was performed for ACS. Twenty-two patients presented with pancreatic necrosis; in those cases, a step-up approach was initiated. Among the patients with pancreatic necrosis, eight patients underwent retroperitoneoscopy and pancreatic necrosis debridement. Four patients underwent a combination of retroperitoneoscopy for pancreatic necrosis and decompressive laparotomy for ACS (Fig. 2). Oral intake was reinitiated as soon as possible according to the patient’s condition and used instead of parenteral feeding. Naso-jejunal feeding tubes were placed in nine patients [22]. Abdominal pressure was routinely monitored according to the bladder method, and the World Society for the Abdominal Compartment Syndrome recommendations were implemented in accordance with the measured intra-abdominal pressure [14, 23]. All patients with ACS required a decompressive laparotomy. A subcostal laparotomy was performed for ACS 10 days after the onset of SAP (5; 20 days). Four patients died during the study period (9.1%). The cause of death was intra-operative massive uncontained bleeding associated with open retropancreatic drainage while performing a decompressive laparotomy for ACS 48 h after ICU admittance in one patient. One patient died on day 5 after the onset of SAP due to the failure of respiratory management in the context of ACS. The cause of death in one patient on the 11th was severe abdominal sepsis due to right colon ischaemia. One patient died on day 6 as a consequence of ACS. All patients who died presented with multi-organ failure and systemic inflammatory response syndrome. The median ICU stay was 8 days (4.5; 19.5), and the median hospital stay was 15 days (10; 23).
Discussion
The results of our study revealed that patients with SAP may be treated in a low-volume hospital as long as a multidisciplinary approach and best practice guidelines are applied. We found a mortality rate of 9.1% in patients with SAP, which is similar to the rates previously reported [2, 4, 24]. Severe acute pancreatitis is associated with substantial morbidity and mortality and is a costly disease. There is a trend towards implementing a step-by-step approach to treating SAP to reduce morbidity, mortality and cost [6, 12, 25]. Mortality has been shown to be reduced in patients who have undergone less-invasive approaches compared to previous strategies [26]. Identifying patients at high risk of mortality who need early ICU management is also of paramount importance [20, 21]. Similarly, the treatment of pancreatic necrosis has evolved from open necrosectomy to a less aggressive surgical strategy defined as step-up approach [7, 27, 28]. We defined a step-by-step flow (diagram 1) starting with the least aggressive procedure available. Upon patient evolution, supplementary procedures were considered. If the patient’s clinical and radiological conditions did not evolve satisfactorily, percutaneous drains were inserted for both intra-abdominal and retroperitoneal infected fluid collections with ultrasound or CT scan guidance [29, 30]. Drains were replaced if necessary. In the case of abdominal infected fluid collections or retroperitoneal infected necrosis at the onset of sepsis, an aggressive approach was initiated. In patients with walled necrosis or retroperitoneal abscess without improvement after percutaneous drain, retroperitoneoscopy and necrosectomy were performed [31]. In those cases, access to the retroperitoneal space was guided by a percutaneous drain placed under CT scan guidance in the area identified as being the most accessible. Drains for continuous lavage were inserted according to this procedure. Although endoscopic management for pancreatic necrosis has been recently used depending on the location of the pancreatic necrosis, it is still not the standard of care [32,33,34]. We performed this procedure in one patient. Intra-abdominal hypertension, which is relatively common in patients with SAP, may lead to ACS. This situation is life-threatening and has to be addressed to prevent deleterious effects. In our group of patients, abdominal pressure was routinely monitored and specific management was initiated as needed [14]. Once ACS was diagnosed, medical treatment was initiated with hollow viscera decompression by means of a naso-gastric or rectal catheter or colonoscopy. Percutaneous drainage was used to decrease the intra-abdominal volume whenever intra-abdominal fluid collections were identified. If ACS did not respond to a non-surgical approach, decompressive laparotomy with temporary abdominal closure was performed [35]. Some patients presented with abdominal sepsis related to colon ischaemia and/or hollow viscera perforation. In those circumstances, urgent surgical treatment was performed. We prevented access to the retroperitoneal space to minimize the risk of massive retroperitoneal bleeding if walling off of the necrosis was not assured. Early oral nutrition has been shown to have beneficial effects in terms of morbidity and mortality among patients with severe acute pancreatitis [6]. Oral intake was promoted as soon as possible, and a naso-jejunal feeding tube was used whenever oral intake was not sufficient. Determining whether hospital volume has an effect on mortality for patients with AP is an important issue. Cut-off points to establish hospital volume related to AP have been described by different authors. Singla et al. defined high-volume hospitals for AP, based on a nationwide US inpatient sample, as those that admit more than 118 patients a year. They concluded that high-volume hospitals admit the most patients with acute pancreatitis compared with low-volume hospitals, and those hospitals had shorter lengths of stay, lower hospital charges and lower mortality rates; however, they did not stratify patients according to their severity of disease [17]. In a Japanese study with 7007 patients from 776 hospitals, Murata et al. categorized hospital volume based on the number of cases with SAP during the study period into low volume (16 cases). Patient data were corrected according to the severity of AP. They found that hospital volume influenced the clinical outcomes in both patients with mild AP and those with SAP [18]. That study did not use standard severity scores for AP in all cases. Even after the bias related to patient distribution was balanced, that study showed that elderly patients were more frequently treated in low-volume hospitals than in high-volume hospitals. In another Japanese study using a Japanese nationwide administrative database, in-hospital mortality, length of stay and total costs for patients with AP were analysed [36]. Severity was adjusted for mainly based on the Japanese Severity Scoring System. That study proposed that despite shorter hospital stays in higher volume hospitals, no volume–outcome relationship was evident for AP and that further evidence was required to justify the volume-based selective referral of patients with AP. A limit for the highest volume quartile of 14 cases of SAP per hospital-year was proposed in a Taiwanese study [37]. Increased hospital volume was associated with a reduced risk of in-hospital mortality after adjusting for patient and hospital characteristics. Interestingly, they observed that the volume effect on in-hospital mortality disappeared regardless of the volume measures after adjusting for treatment covariates. In that study, all patients with SAP requiring admission to the ICU were included except those who only required postoperative ICU admission for mild biliary pancreatitis after cholecystectomy. A plateau in terms of mortality outcomes for hospitals that received ≥ 3 cases year has also been highlighted. This suggests that a relatively low number of patients admitted for SAP are needed to obtain suitable results. Currently, more evidence is still needed to justify hospital volume-based selective referral for patients with SAP [38]. Comparing the volume of SAP patients among hospitals may be quite arbitrary because there is not an accurate definition of a high-volume hospital in relation to AP or SAP. The IAP/APA guidelines do not offer a precise definition of high-volume hospital [6]. Taking into account those previous studies, definition of hospital volume may be based on 11–16 cases of SAP per hospital-year or approximately 118 cases per hospital-year if we consider all patients with AP regardless of severity. Our hospital volume was 44 patients with SAP out of a total of 1075 patients admitted for AP, regardless of the severity of the disease, over a 10-year period. This is equivalent to 4.4 patients with SAP per hospital-year (far below the cut-off point previously presented). On the other hand, considering the total number of patients with SAP regardless of the severity of the disease, the cut-off point is >118 patients per hospital-year. In our study group, the average number of admitted AP patients was 107 patients per year. Standard care for SAP has evolved over the years, and it could be argued that previous studies based on hospital volume have considered strategies that may not have included step-by-step strategies. Although low-volume hospitals are likely to have fewer resources than high-volume hospitals, the implementation of comprehensive and updated protocols for SAP has not been evaluated thus far. This study has several limitations. First, the sample population of patients was small. This was both a cause and a consequence, as our hospital is defined as a low-volume institution. However, it is important to highlight that we provided treatment based on a multidisciplinary decision-making process, best practices based on international guidelines and streamlined processes of care. Second, this study was a retrospective analysis of a prospectively maintained database. As this was not a comparative study, we can only make suggestions. Nevertheless, this study took into account all cases of SAP over a long period. One way to solve the problem of a small number of patients would be to perform such studies in multiple low-volume hospitals and compare the results with those of high-volume hospitals in terms of mortality, morbidity, length of stay and cost. Despite these limitations, we have evaluated the data from a prospectively maintained database in a single low-volume hospital.
Conclusion
Severe acute pancreatitis represents a complex pathology that requires a multidisciplinary approach. Establishing best practice treatments and evidence-based guidelines for SAP may improve outcomes in low-volume hospitals.
References
Peery AF, Dellon ES, Lund J et al (2012) Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143:1179–1187
Roberts SE, Morrison-Rees S, John A et al (2017) The incidence and aetiology of acute pancreatitis across Europe. Pancreatology 17:155–165
Lowenfels AB, Maisonneuve P, Sullivan T (2009) The changing character of acute pancreatitis: epidemiology, etiology, and prognosis. Curr Gastroenterol Rep 11:97–103
Fenton-Lee D, Imrie CW (1993) Pancreatic necrosis: assessment of outcome related to quality of life and cost of management. Br J Surg 80:1579–1582
Banks PA, Bollen TL, Dervenis C et al (2013) Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut 62:102–111
Working Group IAP/APA Acute Pancreatitis Guidelines (2013) IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 13:1–15
van Santvoort HC, Besselink MG, Bakker OJ et al (2010) A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 22:1491–1502
Ranson JH, Rifkind KM, Roses DF et al (1974) Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 139:69–81
Ranson JH (1982) Etiological and prognostic factors in human acute pancreatitis: a review. Am J Gastroenterol 77:633–638
Balthazar EJ, Robinson DL, Megibow AJ et al (1990) Acute pancreatitis: value of CT in establishing prognosis. Radiology 174:331–336
Moreno RP, Metnitz PG, Almeida E et al (2005) SAPS 3-From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 31:1345–1355
Bollen TL, van Santvoort HC, Besselink MG et al (2008) The Atlanta classification of acute pancreatitis revisited. Br J Surg 95:6–21
Tee YS, Fang HY, Kuo IM et al (2018) Serial evaluation of the SOFA score is reliable for predicting mortality in acute severe pancreatitis. Medicine 97:e9654
Kirkpatrick AW, Roberts DJ, De Waele J et al (2013) Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 39:1190–1206
De Waele JJ, Kimball E, Malbrain M et al (2016) Decompressive laparotomy for abdominal compartment syndrome. Br J Surg 103:709–715
George J, Meghij S, Shah J et al (2016) Open pancreatic necrosectomy. A six year experience from a DGH. HPB 18:e782–e787
Singla A, Simons J, Li Y et al (2009) Admission volume determines outcome for patients with acute pancreatitis. Gastroenterology 137:1995–2001
Murata A, Matsuda S, Mayumi T et al (2011) Effect of hospital volume on clinical outcome in patients with acute pancreatitis, based on a national administrative database. Pancreas 40:1018–1023
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Vincent JL, Sharon E, Pearse R et al (2018) Improving detection of patient deterioration in the general hospital ward environment. Eur J Anaesthesiol 35:325–333
Gordo F, Molina R (2018) Evolution to the early detection of severity. Where are we going? Med Intensiva 42:47–49
Petrov MS, Pylypchuk RD, Uchugina AF (2009) A systematic review on the timing of artificial nutrition in acute pancreatitis. Br J Nutr 101:787–793
Malbrain ML, De laet I, Cheatham M (2007) Consensus conference definitions and recommendations on intra-abdominal hypertension (IAH) and the abdominal compartment syndrome (ACS)—the long road to the final publications, how did we get there? Acta Clin Belg 62(44):59
Zerem E (2014) Treatment of severe acute pancreatitis and its complications. World J Gastroenterol 14:13879–13892
Yokoe M, Takada T, Mayumi T et al (2015) Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci 22:405–432
Hamada S, Masamune A, Shimosegawa T (2016) Management of acute pancreatitis in Japan: analysis of a nationwide epidemiological survey. World J Gastroenterol 28:6335–6344
Connor S, Ghaneh P, Raraty M et al (2003) Minimally invasive retroperitoneal pancreatic necrosectomy. Dig Surg 20:270–277
Raraty MG, Halloran CM, Dodd S et al (2010) Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg 251:787–793
Sugimoto M, Sonntag DP, Flint GS et al (2015) A percutaneous drainage protocol for severe and moderately severe acute pancreatitis. Surg Endosc 29:3282–3291
Van Santvoort HC, Bakker OJ, Bollen TL et al (2011) A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 141:1254–1263
Horvath KD, Kao LS, Wherry KL et al (2001) A technique for laparoscopic-assisted percutaneous drainage of infected pancreatic necrosis and pancreatic abscess. Surg Endosc 15:1221–1225
Bakker OJ, van Santvoort HC, van Brunschot S et al (2012) Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 307:1053–1061
Yip HC, Bun Teoh AY (2017) Endoscopic management of peri-pancreatic fluid collections. Gut and Liver 11:604–611
Thorsen A, Borch AM, Novovic S et al (2018) Endoscopic necrosectomy through percutaneous selfexpanding metal stents may be a promising additive in treatment of necrotizing pancreatitis. Dig Dis Sci 63:2456–2465
Van Brunschot S, Schut AJ, Bouwense SA et al (2014) Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 43:665–674
Hamada T, Yasunaga H, Nakai Y et al (2014) Impact of hospital volume on outcomes in acute pancreatitis: a study using a nationwide administrative database. J Gastroenterol 49(1):148–155
Shen Hsiu-Nien, Chin-Li Lu, Li Chung-Yi (2012) The effect of hospital volume on patient outcomes in severe acute pancreatitis. BMC Gastroenterol 12:112
Yokoe Masamichi (2014) Does higher hospital volume improve the patient outcome in acute pancreatitis? J Gastroenterol 49:371–372
Funding
This study has not received grant support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Robin-Lersundi, A., Abella Alvarez, A., San Miguel Mendez, C. et al. Multidisciplinary Approach to Treating Severe Acute Pancreatitis in a Low-Volume Hospital. World J Surg 43, 2994–3002 (2019). https://doi.org/10.1007/s00268-019-05114-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05114-8