Abstract
Background
The Omega-3 (ω-3) polyunsaturated fatty acids (PUFAs) generate bioactive lipid mediators that reduce inflammation. The present study evaluated the effect of SMOFlipid containing ω-3 PUFAs on wound healing.
Methods
Rats were divided into a SMOFlipid (SMOF) group and a 0.9% saline (placebo) group, with eight rats in each group. Wound excision was performed on the dorsal surface of each rat. In the SMOF group, 1 gm/kg SMOFlipid was dissolved in 3 mL saline as a treatment; in the placebo group, 3 mL saline was prepared as a treatment. The treatments were administered intravenously at an initial rate of 0.2 mL/kg body weight/h immediately after wounding, for 72 h. Blood samples were collected for white blood cell, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-10 measurements at the baseline and at 1, 6, 12, 24, 48, and 72 h after intervention. Wound areas were measured over a 2-week period after excision, and a histological examination was performed.
Results
Compared with the placebo group, SMOFlipid supplementation engendered significant decreases in the wound area on day 3 (78.28 ± 5.25 vs. 105.86 ± 8.89%), day 5 (72.20 ± 4.31 vs. 96.39 ± 4.72%), day 10 (20.78 ± 1.28 vs. 39.80 ± 10.38%), and day 14 (7.56 ± 0.61 vs. 15.10 ± 2.42%). The placebo group had a higher TNF-α level than the SMOF group at 72 h. The IL-10 level was higher in the SMOF group than in the placebo group at 48 h. Histological analysis revealed a higher rate of fibroblast distribution and collagen fiber organization in the SMOF group (P = 0.01).
Conclusion
SMOFlipid enriched in ω-3 PUFA accelerates wound healing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wound healing is divided into inflammatory response, proliferation, and maturation phases [1,2,3]. Transition from the inflammatory phase (within 3 days after the initial wounding) to the proliferative phase (between day 4 and day 14) is a critical stage during healing [1,2,3]. When tissue is first wounded, the ruptured cell membranes release inflammatory cytokines that cause the vessels in the wound bed to contract, and a clot is formed to prevent hemorrhage [1]. Once hemostasis has been accomplished, blood vessels dilate to allow white blood cells (WBCs), inflammatory cells, and nutrients to reach the wound bed, thus protecting the wound against attacks by pathogens [1].
Lipid emulsions (LEs), which are rich in Omega-3 (ω-3) fatty acids, have several biologically active components [4,5,6]. Researchers have indicated that LEs can also be considered to be pharmacological agents and to serve as substrates for mediators that have functions in immune responses by nutrition [4, 5]. Proinflammatory cytokine synthesis and activity are affected by the concentration of ω-3 fatty acids. The active involvement of fatty acids in epidermal health proves that the systematic addition of ω-3 polyunsaturated FAs (PUFAs) is an innovative strategy for improving inflammatory responses [6, 7], and PUFAs are key beneficial sources for the structure and function of cell membranes [5,6,7].
SMOFlipid (SMOFlipid 20%, Fresenius Kabi, Bad Homburg, Germany) is a LE mixture of four different lipid sources: soybean oil, medium-chain triglycerides, olive oil, and fish oil (SMOF). It contains an altered ratio of ω-6:ω-3 fatty acids, and it is beneficial in parenteral nutrition (PN) [7,8,9]. ω-3 fatty acids such as EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) have demonstrable effects on cell membranes and inflammatory processes [7,8,9,10]. SMOFlipids are rich in ω-3 fatty acids and inhibit the production of proinflammatory cytokines by activating the interaction between peroxisome proliferator-activated receptors and NF-kappa B [10,11,12]. Studies have indicated that dietary supplements of ω-3 can affect the gene expression of proinflammatory cytokines during the inflammatory phase of wound healing [1, 2]. Therefore, the anti-inflammatory mechanism of SMOFlipids involves the possible inhibition of excessive endothelial activity to reduce the production of proinflammatory mediators [11, 12]. Although ω-3 PUFA supplementation may improve the inflammatory response, whether SMOFlipid supplementation influences wound healing is still unclear [11, 12]. In this study, we investigated the effects of LEs enriched in ω-3 PUFAs on anti-inflammatory properties wound healing in a rat model. We hypothesized that SMOFlipid supplementation accelerates the inflammatory and proliferative phases of wound healing through decreasing the production of proinflammatory cytokines.
Materials and methods
Study design and animal preparation
This study was a placebo-controlled experimental study. Sprague–Dawley rats (aged 13 weeks and weighed 200–250 g) were purchased from the National Laboratory Animal Center (Taipei, Taiwan). The Institutional Review Committee of the Animal Usage Regulation Committee of Tzu Chi University approved all animal study protocols (Protocol #101023). The sample size was calculated under the consideration of a 20% difference in wound area between the ω-3 PUFA supplementation and placebo groups, as well as an estimated SD of 9.6% based on data from a previous study [13]. Therefore, with an alpha of 0.05 and a power of 80%, the final sample size chosen was eight rats per group. For the surgical procedure, rats were anesthetized and a PE-50 catheter was inserted into the femoral vein for intravenous injection. An excisional wound was created on a thin rectangular piece of skin (20 × 10 mm in diameter) on the dorsum of each rat. After the surgical procedure was completed, the rats were randomly assigned to the two groups; these groups were systemically administered either SMOFlipid 20% or a placebo (n = 8 in each group) intravenously through an infusion pump immediately after wounding, and they were continuously treated for 72 h.
Administration of lipid emulsion
The composition of the LE used in this study was SMOF lipid 20%, comprising soybean oil (30%), MCTs (30%), olive oil (25%), and fish oil (15%) [7]. The dose criterion was 2 g fat/kg body weight (bw)/day, corresponding to 10 mL/kg bw/day. The infusion rate was 0.2 mL SMOFlipid/kg bw/h [9]. During the administration of intravenous solutions, rats were housed in metabolic cages to prevent coprophagia. After wounding, the rats in the SMOF group (n = 8) were infused with 20% of SMOF at 0.2 mL/kg bw/h for 72 h by using an infusion pump. The rats in the placebo group (n = 8) were infused continuously with NS at 0.2 mL/kg bw/h after wounding, for 72 h. The intervention point was at 72 h to reduce the bacterial contamination of lipid fluids [9, 10].
Measurement of wound area
On the basis of the wound healing process, the 14th day after wounding was the primary end point of this experiment. Wound healing was considered as the primary outcome measurement, and biomarkers of inflammation and the histological examination of wound tissue were chosen as the secondary outcomes. Wound measurements were conducted at the baseline and 5, 7, 10, 12, and 14 days after intervention. The full-thickness wound area was measured under the same conditions with a digital camera (Nikon D7000, Japan), and subsequently, an AxioVision SE64 microscope was used to measure the wound size. Wound closure was reported as the percentage area of the wound that had closed, and it was calculated as follows: open area of the healing wound on a given day/open area of the original wound × 100 for the indicated days.
Blood sample analysis
Blood samples from the femoral artery were collected at the baseline and at 1, 6, 12, 24, 48, and 72 h after intervention. The WBC count of the samples was then measured (Sysmex K-1000, USA). After the WBC examination, the samples were centrifuged immediately at 3000g for 10 min. The serum was stored at −20 °C for the subsequent determination of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-10 concentrations.
Histological examination of wound tissue
Full-thickness incisional wounds were dissected for histological examination. The wound tissues harvested on day 14 were fixed in phosphate buffered saline with 4% paraformaldehyde for 24 h. After the paraffin tissue was sectioned, the harvested wound tissues were cut in the middle, perpendicular to the incision line, and the paraffin was embedded and sliced to a thickness of 2 mm. Tissue sections were stained with hematoxylin and eosin (H&E). After H&E staining, tissue sections of the central wound area were observed with a fixed magnification (40–100×) under a microscope (Leica TCS SP2, Germany). In this study, the histological examinations, including the number of acute and chronic inflammatory infiltrations, granulation tissue maturation, collagen deposition, epithelial regeneration, and neovascularization, were performed using Abramov’s histological scoring system [14,15,16]. Two independent investigators were trained to use the scoring system and were blinded to the allocation groups.
Enzyme-linked immunosorbent assay
The TNF-α, IL-6, and IL-10 concentrations of the culture supernatant were evaluated by enzyme-linked immunosorbent assay (ELISA, Endogen, Rockford, IL, USA). All work standards were prepared in accordance with the instructions of the manufacturer. The reaction was measured at wavelengths of 450 and 540 nm with an ELISA reader (Sunrise, Tecan Co., Grödingen, Austria).
Statistical analysis
The independent samples t test was used for statistical comparisons. A P value of less than 0.05 was considered statistically significant. Data are presented as mean ± standard deviation. Statistical analysis was performed using SPSS software version 18 (SPSS Inc., Chicago, Illinois, USA).
Results
Influence of intravenous lipid emulsion on wound closure rate
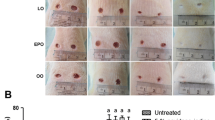
The changes in wound size are shown in Fig. 1a. The SMOF treatment accelerated the healing process compared with the performance of the placebo group by reducing the surface area of the wound by 20–25% on day 3 of treatment. Macroscopic observations indicated that wounds treated with SMOF had reduced inflammation, redness, and thickness, compared with those treated with the placebo. Wound areas in the placebo group exhibited more severe swelling, compared with those in the SMOF group. The data relating to wound diameter indicated a significant delay in wound healing in the placebo group compared with the SMOF group, especially on days 3, 5, 10, and 14 (P < 0.05) (Fig. 1b).
Progressive changes in morphology and size of the wound. Macroscopic observation showed that there was a delay in reepithelialization as well as prolonged inflammation and a delay in granulation tissue formation in the placebo group (a). Changes in wound size (b) until wound closure are expressed as a percentage of the initial wound area. *P < 0.05 indicates the SMOF group compared with the placebo group
Levels of WBC and inflammatory cytokines
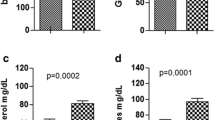
The levels of WBC were similar in both groups before wound excision. After wound excision, the WBC levels of both the SMOF and placebo groups decreased at 1 h and then gradually recovered to the baseline level at 6 h. After 12 h, a secondary decrease was observed, followed by a decrease at 72 h. Subsequently, a gradual reduction was observed (Fig. 2a). The placebo group exhibited higher WBC levels at 1, 12, and 24 h, compared with SMOF group, indicating a significant difference between the two groups (P < 0.05).
Changes in white blood cell values (a), tumor necrosis factor (TNF-α) levels (b), changes in interleukin 6 (IL-6) levels (c), and Changes in interleukin 10 (IL-10) levels (d) after excisional wound. “Pre” indicates baseline measures before wounding. *Indicates the SMOF group compared with the placebo group and P < 0.05
In both groups, the serum levels of TNF-α decreased in the early phase after wound excision, but they increased again at 72 h in the placebo group (Fig. 2b). At 72 h, the TNF-α level was significantly higher in the placebo group (41.00 ± 13.63 pg/mL) than in the SMOF group (3.87 ± 3.82 pg/mL; P < 0.05). The levels of IL-6 decreased at 24 h, and this was followed by a subsequent increase at 72 h (Fig. 2c). The SMOF group exhibited higher IL-6 levels before surgery and at 24 and 72 h after surgery; however, differences between the levels in each group were deemed nonsignificant. IL-10 levels were slightly similar in the two groups before wound excision. After surgery, a decrease in IL-10 levels was observed in both groups, followed by an increase in the SMOF group at 48 h, thereby indicating significant differences (P < 0.05) between the two groups (Fig. 2d).
Histological analysis
Histological comparisons of wounds in both groups at postoperative day 14 after wound excision are shown in Fig. 3. Regarding the inflammatory cells, the placebo group exhibited neutrophil surrounding the tissue and less angiogenesis (Fig. 3a, b). Compared with the SMOF group, the granulation tissue contained increased fibroblasts arranged irregularly and the arrangement of collagen fibers was decreased in the placebo group (Fig. 3c). The SMOF group demonstrated greater healing ability, such as revascularization of the wound that proceeded in parallel with fibroplasia (Fig. 3d), which signaled the initiation of the formation of granulation tissue. The results of the histopathological evaluation are presented in Fig. 4. In this study, the SMOF group had a significantly increased collagen deposition at the wound site relative to the placebo group (3.00 ± 0.00 vs. 2.00 ± 0.40, P = 0.01). The acute inflammation score was higher for the placebo group than for the SMOF group (1.00 ± 0.40 vs. 0.33 ± 0.21, P = 0.15). The granulation tissue, fibroblast, reepithelialization, and neovascularization levels in the SMOF group were also higher than those in the placebo group, but there were no significant differences between the two groups.
Histological comparisons of wounds posttreatment with SMOF and placebo. Histological sections from the placebo (a, c) and SMOF (b, d) groups (magnification ×100 and ×400). Black narrow arrows point to the sites of vessels, white arrows point to the sites of vessels collagen fibers, black broad arrows point to the sites of vessels fibroblasts
Mean values of histopathology scores of wound healing among SMOF and placebo groups. AI indicates acute inflammation, AGI indicates amount and maturation of granulation tissue, GTFM indicates Granulation tissue fibroblast maturation, CD indicates collagen deposition, RE indicates reepithelialization, and NV indicates neovascularization. *P < 0.05 indicates the SMOF group compared with the placebo group
Discussion
Studies have reported that fish oil, which is rich in ω-3, may exert potential beneficial effects on wound healing in various ways [1, 3, 9, 19, 21]. Our data reveal that wound healing was improved in the SMOF group. The placebo group exhibited an increase in the cellularity of the tissue around the wound site, which was considerably greater than that of the SMOF group; furthermore, the wound areas of the SMOF group were considerably contracted, compared with those of the placebo group on day 14. McDaniel et al. [17] investigated the effects of the administration of fish oil compared with mineral oil on healthy adults. The group that received the ω-3 supplement exhibited greater epithelialization of blisters than did the control group [17]. These findings are similar to our study results: SMOFlipid may have a positive effect on early skin epithelialization, thus reducing the swelling around the wound site and promoting faster healing. When the wound tissue in the SMOF group was analyzed 14 days after wounding, some new blood vessels in the surface and deep areas of the granulation tissue were observed. Therefore, we suspect that SMOFlipid supplementation has a positive effect on the inflammatory and proliferation phases of wound healing.
In addition, wound healing, immune competence, and skin integrity all require essential fatty acids [17, 18]. In the present study, the SMOF group exhibited a decrease in proinflammatory cytokine TNF-α (significant differences at 72 h) and WBC levels (significant differences at 1, 9, 12, and 24 h), compared with the placebo group. Thus, lipid chemoattractants may affect the leukocytic infiltrate and the inflammatory cell function, possibly due to their component fatty acids [18,19,20,21,22,23]. Although the provided SMOFlipid decreased the number of TNF-α and WBC counts, it did not lower the cytokine IL-6 levels. Notably, IL-6 is traditionally considered to be a proinflammatory cytokine [24,25,26]. Regarding wound healing, IL-6 exhibits both pro- and anti-inflammatory properties [27,28,29]. Previous studies have reported that IL-6 levels were significantly increased after tissue trauma, and a positive correlation has been identified between IL-6 levels and collagen production [23,24,25,26,27]. We were unable to establish the exact relationship between SMOFlipid and IL-6 levels; there are still numerous unknown fields that require further study.
IL-10 is widely recognized as an immunosuppressive cytokine, due to its suppression of inflammation in wound healing [29, 30]. In this study, the SMOF group also demonstrated an increase in anti-inflammatory cytokine IL-10 levels, compared with the placebo group, and there were significant differences between the two groups. Several clinical trials have been performed to demonstrate the ability of IL-10 overexpression to regenerate wound healing and induce scarless healing [29, 30]. The role of the regenerative effects of IL-10 is considered to be the inhibition of the synthesis of proinflammatory cytokines and suggests that the initiation of fish oil-supplemented LE beneficially alters the lipid profile in plasma, modulates immune function, increases IL-10 levels, and regulates inflammatory responses [30, 31]. Therefore, these results indicate that SMOFlipid has anti-inflammatory effects on cytokine secretion.
Although the differences were deemed nonsignificant, when compared with those of the placebo group, the IL-6 levels were higher in the SMOF group at 24 and 72 h after wound excision. Notably these findings did not support our original hypotheses, and further research in this area is required [21, 27]. These identified trends might have produced statistically significant results if a larger sample size had been utilized. In addition, it would have been beneficial to conduct histological assessments of the skin biopsies of other groups of rats during the early postexcision phase (e.g., on days 3 and 5) and to apply Masson’s trichrome stains for the detection of collagen fibers.
In summary, the results demonstrate that the levels of reepithelialization were higher in the SMOF group than in the placebo group, thus significantly improving the inflammatory and proliferative phases of wound healing. The SMOF group also demonstrated increasing trends in the IL-10 levels. Compared with those of the placebo group, the administration of SMOFlipid caused considerable decreases in the levels of WBCs and the proinflammatory cytokine TNF-α. The findings of this study support the proposition that SMOFlipid affects the proinflammatory cytokine TNF-α that regulates the wound healing process. SMOFlipid supplementation might be valuable for improving standard forms of clinical therapy, especially in hyperinflammatory conditions, as well as acting as an adjunct therapy for treating surgical wounds.
References
Klein KC, Guha SC (2014) Cutaneous wound healing: current concepts and advances in wound care. Indian J Plast Surg 47(3):303–317
Soleimani Z, Hashemdokht F, Bahmani F et al (2017) Clinical and metabolic response to flaxseed oil omega-3 fatty acids supplementation in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabetes Complicat 31(9):1394–1400
de Castilho TJ, Campos AC, Mello EV (2015) Effect of omega-3 fatty acid in the healing process of colonic anastomosis in rats. Arg Bras Cir Dig 28(4):258–261
Baena-Gómez MA, Aguilar MJ, Mesa MD et al (2015) Changes in antioxidant defense system using different lipid emulsions in parenteral nutrition in children after hematopoietic stem cell transplantation. Nutrients 7(9):7242–7255
Ren T, Cong L, Wang Y et al (2013) Lipid emulsions in parenteral nutrition: current applications and future developments. Expert Opin Drug Deliv 10(11):1533–1549
Klek S, Chambrier C, Singer P et al (2013) Four-week parenteral nutrition using a third generation lipid emulsion (SMOFlipid)—a double-blind, randomised, multicentre study in adults. Clin Nutr 32(2):224–231
Uthaya S, Liu X, Babalis D et al (2016) Nutritional Evaluation and Optimisation in Neonates: a randomized, double-blind controlled trial of amino acid regimen and intravenous lipid composition in preterm parenteral nutrition. Am J Clin Nutr 103(6):1443–1452
Dai YJ, Sun LL, Li MY et al (2016) comparison of formulas based on lipid emulsions of olive oil, soybean oil, or several oils for parenteral nutrition: a systematic review and meta-analysis. Adv Nutr 7(2):279–286
Tian H, Yao X, Zeng R et al (2013) Safety and efficacy of a new parenteral lipid emulsion (SMOF) for surgical patients: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev 71(12):815–821
Bolisetty S, Osborn D, Sinn J, Lui K (2012) Standardised neonatal parenteral nutrition formulations—an Australasian group consensus. BMC Pediatr 14:48–59
Li H, Ruan XZ, Powis SH et al (2005) EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int 67(3):867–874
Calder PC, Yaqoob P (2009) Understanding omega-3 polyunsaturated fatty acids. Postgrad Med 121(6):148–157
Baldan CS, Masson IF, Esteves Junior I et al (2015) Inhibitory effects of low-level laser therapy on skin-flap survival in a rat model. Plast Surg 23(1):35–39
Abramov Y, Golden B, Sullivan M et al (2007) Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen 15(1):80–86
Khoshmohabat H, Dalfardi B, Dehghanian A et al (2016) The effect of CoolClot hemostatic agent on skin wound healing in rats. J Surg Res 200(2):732–737
Kamer E, Recai Unalp H, Gundogan O et al (2010) Effect of ascorbic Acid on incisional wound healing in streptozotocin-induced diabetic rats. Wounds 22(2):27–31
McDaniel JC, Massey K, Nicolaou A (2011) Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds. Wound Repair Regen 19(2):189–200
Kendall AC, Nicolaou A (2013) Bioactive lipid mediators in skin inflammation and immunity. Prog Lipid Res 52(1):141–164
Jafari Naveh HR, Taghavi MM, Shariati M et al (2011) Both omega-3 and omega-6 polyunsaturated fatty acids stimulate foot wound healing in chronic diabetic rat. Afr J Pharm Pharmacol 5(14):1713–1717
Lee RP, Wang D, Lin NT et al (2002) Physiological and chemical indicators for early and late stages of sepsis in conscious rats. J Biomed Sci 9(6 Pt 2):613–621
McDaniel JC, Belury M, Ahijevych K et al (2008) Omega-3 fatty acids effect on wound healing. Wound Repair Regen 16(3):337–345
Lu Y, Tian H, Hong S (2010) Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing. J Lipid Res 51(5):923–932
McDaniel JC, Ahijevych K, Belury M (2010) Effect of omega-3 oral supplements on the omega-6/omega-3 ratio in young adults. West J Nurs Res 32(1):64–80
Fontes JA, Rose NR, Čiháková D (2015) The varying faces of IL-6: from cardiac protection to cardiac failure. Cytokine 74(1):62–68
Choi JH, Jun JH, Kim JH et al (2014) Synergistic effect of interleukiomega-6 and hyaluronic acid on cell migration and ERK activation in human keratinocytes. J Korean Med Sci 29(Suppl 3):S210–S216
Muldoon MF, Laderian B, Kuan DC et al (2016) Fish oil supplementation does not lower C-reactive protein or interleukiomega-6 levels in healthy adults. J Intern Med 279(1):98–109
Hankenson KD, Watkins BA, Schoenlein IA et al (2000) Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukiomega-6 production. Proc Soc Exp Biol Med 223(1):88–95
Stenvinkel P, Ketteler M, Johnson RJ et al (2005) IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int 67(4):1216–1233
Balaji S, King A, Marsh E et al (2015) The role of interleukin-10 and hyaluronan in murine fetal fibroblast function in vitro: implications for recapitulating fetal regenerative wound healing. PLoS ONE 10(5):e0124302
Kieran I, Knock A, Bush J et al (2013) Interleukin-10 reduces scar formation in both animal and human cutaneous wounds: results of two preclinical and phase II randomized control studies. Wound Repair Regen 21(3):428–436
de Miranda Torrinhas RS, Santana R, Garcia T et al (2013) Parenteral fish oil as a pharmacological agent to modulate post-operative immune response: a randomized, double-blind, and controlled clinical trial in patients with gastrointestinal cancer. Clin Nutr 32(4):503–510
Acknowledgements
This work was partially supported by the Tzu Chi University (TCIRP 101002-01Y1). This manuscript was edited by Wallace Academic Editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors declare any conflict of interest.
Rights and permissions
About this article
Cite this article
Peng, YC., Yang, FL., Subeq, YM. et al. Lipid Emulsion Enriched in Omega-3 PUFA Accelerates Wound Healing: A Placebo-Controlled Animal Study. World J Surg 42, 1714–1720 (2018). https://doi.org/10.1007/s00268-017-4404-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-4404-x