Abstract
Background
Prevalence of diabetes in surgical patients is 10–40%. It is well recognized that they have higher rates of complications, and longer stays in hospital compared to patients without diabetes. Enhanced recovery after surgery (ERAS) is an evidence-based multimodal surgical care pathway that improves postoperative complications and length of stay in patients without diabetes. This review evaluates the evidence on whether individuals with diabetes would benefit from ERAS implementation.
Methods
MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL) and EMBASE searched with no language restrictions applied. Conference proceedings and bibliographies were reviewed. Experts in the field were contacted, and www.clinicaltrials.gov searched for ongoing trials.
Selection criteria
Randomized controlled trials (RCT) looking at individuals with diabetes undergoing surgery randomized to ERAS® or conventional care. Non-randomized controlled trials, controlled before–after studies, interrupted time series, and cohort studies with concurrent controls were also considered. Two authors independently screened studies.
Results
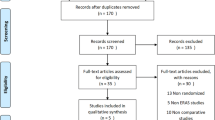
The electronic search yielded 437 references. After removing duplicates, 376 were screened for eligibility. Conference proceedings and bibliographies identified additional references. Searching www.clinicaltrials.gov yielded 59 references. Contacting experts in the field identified no further studies. Fourteen full articles were assessed and subsequently excluded for the following reasons: used an intervention other than ERAS®, did not include patients with diabetes, or used an uncontrolled observational design.
Conclusions
To date, the effects of ERAS® on patients with diabetes have not been rigorously evaluated. This review highlights the lack of evidence in this area and provides guidance on design for future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Diabetes is a chronic disease with an estimated prevalence of 7.2–11.4% worldwide [339–536 million], and an expected rise in these numbers over the upcoming decades [1]. People with diabetes are more likely to require surgery compared to people without diabetes, and prevalence of diabetes in surgical patients is estimated to be 10–15% [2–4] and up to 40% in those undergoing bariatric surgery [5–7]. It is well recognized that people with diabetes are a high-risk surgical population with longer hospital stays, higher healthcare resource utilization, and greater perioperative morbidity and mortality [8–10].
Enhanced recovery after surgery (ERAS®) is an evidence-based multimodal surgical care pathway first published in 2005 [11, 12] and has been shown in multiple RCTs to reduce LOS by 30%, and reduce postoperative complications by up to 50% [13–18]. It includes 22 components of pre-, intra- and postoperative care, with all being described as “Strong” recommendations as per the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework [19]—Table 1 [16, 17, 20, 21]. The protocol was last updated in 2012 [20].
Whether patients with diabetes should be included in ERAS® has been a contentious issue [22, 23], resulting in varying clinical practice and guideline recommendations [17, 22, 24, 25]. There are hypothetical risks to carbohydrate loading (one of the key preoperative elements of ERAS®) in individuals with diabetes (such as aspiration pneumonia due to delayed gastric emptying, and hyperglycemia and its sequelae) and it is uncertain whether this may negate any beneficial effects from the other elements of ERAS®. Individuals with diabetes are at risk for delayed gastric emptying. Chronically elevated glucose can lead to autonomic neuropathy and intrinsic nervous system dysfunction in multiple cellular targets, such as neurons producing nitric oxide, interstitial cells of Cajal in the gut, and gut hormone levels [26–28]. Those affected have deranged proximal gastric accommodation and contraction post solid meals and reduced frequency of antral contractions. Acute hyperglycemia (>11.1 mmol/l) could also result in delayed gastric emptying; the rise in glucose decreases stomach and small bowel contractility and stimulates localized pyloric contraction [29, 30]. Another concern with hyperglycemia is its association with postoperative complications [31, 32]. Substantial evidence supports this association, particularly with postoperative infections, with hyperglycemia resulting in a relative state of immunosuppression mediated through multiple immunological pathways (e.g., white blood cells and neutrophil activity, inflammatory cytokine cascade, microvasculature, and reactive oxygen species production) [33].
We conducted this systematic review to evaluate the state of the evidence base for the use of the ERAS® protocol in individuals with diabetes undergoing surgery.
Methods
As per the recommendations of the Cochrane Effective Practice and Organization of Care Group (EPOC) for evaluating healthcare interventions, we included randomized controlled trials (RCTs), subgroups of RCTs in individuals with diabetes undergoing elective surgery, and non-randomized studies with more robust methodology including non-randomized controlled trials (NRCT), controlled before–after (CBA) studies, interrupted time series (ITS), and cohort studies with concurrent controls [34]. The intervention was the full suite of all 22 elements of ERAS®. Non-ERAS protocols were excluded (e.g., fast-track, enhanced recovery program) due to lack of standardization of these protocols and heterogeneity.
Types of outcome measures
Outcomes
Our primary outcome of interest was length of stay (LOS) in hospital. Secondary outcomes included postoperative complications, 30-day readmission and complication rates, episodes of hyperglycemia (glucose >14 mmol/l preoperatively or postoperatively, since this level has been shown to be associated with higher rates of postoperative infections in individuals with diabetes undergoing non-cardiac surgery) [32].
Search methods for identification of studies
Electronic searches
Studies were identified by searching MEDLINE(R) In-Process and Other Non-Indexed Citations and MEDLINE(R) 1946 to Present, EBM Reviews—Cochrane Central Register of Controlled Trials June 2016, EMBASE 1996 to 2016 Week 25. There was no language restriction (see Appendix 1 in supplementary material online). The search was run in Ovid Databases on June 22 2016, and duplicates were removed. Searches were limited from year 2000 to the current year, as the first ERAS protocol was not published until 2005 [11, 12].
Other resources
Additional studies were identified and included by searching conference proceedings of surgical and anesthesiology societies, and the bibliographies of review articles and trials of identified studies. Experts in the area were contacted, and www.clinicaltrials.gov was also searched for ongoing trials.
Data collection and analysis
All retrieved study titles were scanned independently for inclusion by two authors; Zaina Albalawi (ZA) and Michael Laffin (ML), using a standardized form. Disagreement was solved by consensus between them, and full articles were retrieved for those thought to be potentially relevant. Abstracts from conference proceedings were included if the authors were able to provide details for the full study. RevMan (version 5.3) was used. For NRS, additional data would be collected using the data collection form developed by the Cochrane Collaboration [35]. This provides a standardized tool for collecting data on study designs, potential sources of confounding, and risk of bias [36, 37].
Results
The electronic search yielded 437 references. After removing duplicates, 376 remained for screening for eligibility. Searching bibliographies of reviews, conference proceedings, and ERAS guidelines identified additional eight references. Searching www.clinicaltrials.gov yielded 59 references. Contacting experts in the field identified no further studies. The study flow diagram illustrates the search results (Fig. 1) [38]. Fourteen full articles were retrieved, and subsequently excluded as they used an intervention other than ERAS® (n = 10), they did not include patients with diabetes (n = 2) or used an uncontrolled before–after design (n = 2). One of the latter studies included subjects with diabetes among others without diabetes [39]. This included only eight subjects (10.4%) with diabetes in the ERAS intervention group, and 15 (13.8%) in the conventional care group. The author was contacted to provide individual data to further analyze this group, but we were unable to obtain this.
PRISMA study flow diagram [38]
Discussion
This review did not identify any randomized controlled trials or observational studies meeting the EPOC criteria evaluating the effects of ERAS® in patients with diabetes. To the best of our knowledge, this is the first systematic review addressing this question. It highlights the lack of evidence in this area, which has lead to varying practices at sites where ERAS® is implemented [25]. It is likely that all potentially relevant studies were identified in this review with the sensitive search strategy used, as well as the methods utilized to identify grey literature.
The question that remains unanswered is whether patients with diabetes enrolled in ERAS® would observe similar improvements in postoperative outcomes as those reported in trials of patients without diabetes, or whether those would be negated by the potential risks of hyperglycemia and delayed gastric emptying from the carbohydrate load. It is also unknown whether implementing ERAS and monitoring adherence would be cost effective in individuals with diabetes [13, 40]. On the contrary, it is well recognized (and supported by an extensive body of evidence) that individuals with diabetes are at higher risk for morbidity and mortality postoperatively across various types of surgeries [8, 9, 41–46]. Within the context of enhanced recovery programs, results from a small number of observational studies in individuals with diabetes are inconsistent. The only study using ERAS® was Luther and colleagues where they analyzed 18 patients in the diabetes group and 125 in the non-diabetes group. Both groups were scheduled to undergo an elective major colorectal procedure and were enrolled in ERAS®. They found the median length of stay in the diabetes group to be significantly longer at 7 days with an interquartile range of 5–15.5 days compared to 5 days in the non-diabetes group (interquartile range 4–7.5 days) P = 0.041 [47]. It is unknown as well given the study design—whether the 7-day LOS observed in the diabetes group is different than the expected LOS outside of ERAS® implementation. This study was excluded from our systematic review because the control arm did not include individuals with diabetes and was not “conventional care.” The second study used a fast-track protocol in primary total hip and total knee arthroplasty and found no association between diabetes and postoperative morbidity [48].
Revisiting the clinical significance of gastroparesis preoperatively in individuals with diabetes
Although gastroparesis is one of the main potential barriers to carbohydrate loading—and including individuals with diabetes in ERAS®, it is not as common as previously thought [28, 49–51]. In a recent population-based study, gastroparesis was found to be relatively uncommon with a cumulative incidence of 1% in patients with type 2 diabetes versus 0.2% in controls over 10 years [52]. Previous reports of gastroparesis prevalences of 30–50% were overestimates, biased as they were derived in high-risk cohorts from tertiary centers [53, 54]. A large population-based study found this to be only 5–12% in individuals with diabetes [28]. The most common cause of gastroparesis is in fact idiopathic in over one-third of patients [51, 55]. Even in the presence of gastroparesis, slower movement of solid food (rather than liquids) from the stomach is the hallmark of this condition [30, 50, 51].
Two studies have assessed a liquid carbohydrate load in individuals with diabetes, and their results would suggest that harm is unlikely given complete gastric emptying at ≤180 min: (1) Jones et al. administered a liquid drink containing 15 g of dextrose in subjects with variable diabetes control [n = 86, median HbA1c 9.3% (3.6–16%)], majority on insulin. Gastric half-emptying time (T50) was not delayed in 72% of the participants, and all participants had emptied the liquid by about 60 min compared to about 40 min in healthy controls. Oral hypoglycemic agents were held, patients on insulin (76.7%) administered their usual doses ~20 min prior to consuming the drink [56], (2) the second study by Gustafsson and colleagues used a 50 g carbohydrate drink in a better controlled group (n = 25, mean HbA1c 5.6 and 6.8% in non-insulin and insulin treated subjects, respectively). Participants’ usual oral hypoglycemic medications and insulins were administered at their usual doses immediately prior to consuming the drink. They found no signs of delayed gastric emptying (T50 49.8 ± 2 min compared to 58.6 ± 3.7 min), and gastric emptying was complete by 180 min [57]. This would suggest the acceptable 2–3 h time frame for consumption of a liquid load preoperatively by modern fasting guidelines and ERAS®. Furthermore, for passive regurgitation and pulmonary aspiration to occur, gastric content needs to be >200 ml [58, 59]. With the current volumes of carbohydrate delivered at a maximum of 400 ml (50–60 g of carbohydrates), and T50 reported at <1 h from the previous studies, the suggested 2–3 h time frame is conservative. It should also be highlighted that there are other risk factors for aspiration preoperatively, which are far more common than diabetes: anesthesia and airway management [22]. Therefore, in a setting where those two latter factors are controlled (i.e., ERAS®), the risk of aspiration with the carbohydrate load of 400 ml is expected to be low, even in individuals with diabetes and delayed gastric emptying.
ERAS® or other fast-track programs?
The ERAS ® Care System comprises three components and has been tested and implemented as a package in about 40 hospitals in Europe and North America. These components include the (1) ERAS ® Protocol; the (2) ERAS ® Implementation Program, and the 3) ERAS ® Interactive Audit System (EIAS) [12, 60]. There are multiple surgical protocols other than ERAS® such as fast-track protocols and enhanced recovery (ERP) protocols that have been evaluated in surgical patients [48, 61–63]. A number of these studies are limited by the heterogeneity of their protocols, types of surgery, surgical technique, and study design. There is controversy on whether all the ERAS® components are needed to impact postoperative outcomes, or whether a refined “shorter list” would suffice [64–67]. Currently ERAS® is implemented as a package and future research would seem warranted to identify which components of ERAS® have the greatest impact and thereby develop a refined list which would improve allocation of resources to those interventions with the highest impact. Previous attempts to identify which components of ERAS® have a measurable clinical impact of value have been inconsistent and failed to identify a refined list. However, a recent multicenter ERAS® registry data analysis (13 centers from 6 countries between 2008 and 2013) demonstrated that increasing compliance with the ERAS® program and the use of laparoscopic surgery independently improve outcomes [60]. ERAS® stands out among other protocols with its structured perioperative care system—described earlier. More importantly, adherence to ERAS® has recently been shown to be associated with increased 5-year cancer-specific survival after colorectal cancer surgery [68], not reported with any other fast-track or enhanced recovery program.
Implications for practice
Currently, there is a lack of evidence about the impact of ERAS® in surgical patients with diabetes. There is no robust evidence to support any specific recommendations for this population. Clinical judgment (and close monitoring of glucose levels and diabetic medication management) will be required on a case-to-case basis until further evidence is available to inform this decision. It is important to consider the recommended glucose target for the perioperative period, which is 4.4–10.0 mmol/l (80–180 mg/dl) while avoiding hypoglycemia [69, 70]. Basal–bolus insulin regimens are recommended over sliding scale regimens and are associated with better glycemic control and lower postoperative complications [71, 72]. For individuals who cannot take anything by mouth or have minimal food intake postoperatively, basal insulin in combination with a correction bolus is advised [71], although intravenous insulin may be preferred in type 1 diabetes. Specific guidelines on insulin dosing in surgical patients with diabetes are outlined in detail in the Joslin’s evidence-based guidelines available at: https://www.joslin.org/docs/Inpatient-management-of-surgical-patients-with-diabetes-_12-30-2015.pdf [73].
Implications for research
A randomized controlled trial is needed to examine the impact of ERAS® implementation on patients with diabetes, with sufficient numbers of subjects with type 1 diabetes to permit a meaningful sub-analysis. If this is not feasible, then one of the following NRS would be preferred: NRCT, CBA, or an ITS. The ITS design has been recognized as “one of the most effective and powerful of all quasi-experimental designs” [74] and is the best next step if randomization is not possible [75]. Although uncontrolled before–after designs are appealing for their simplicity, their use is discouraged due to their tendency to overestimate benefits of new interventions [76]. Alternatively, a CBA design can be used to compare the before–after effect of implementation of ERAS to a concurrent control group to adjust for trends over time.
In conclusion, our rigorous systematic review highlights the lack of evidence on the effects of ERAS® for surgical patients with diabetes. A policy change in ERAS® implementation is suggested to encourage evaluation when ERAS® is used in individuals with diabetes.
References
International Diabetes Federation (2015) IDF Diabetes Atlas, 7th edn. International Diabetes Federation, Brussels, Belgium. http://www.diabetesatlas.org
Clement S, Braithwaite SS, Magee MF et al (2004) Management of diabetes and hyperglycemia in hospitals. Diabetes Care 27:553–591
Smiley DD, Umpierrez GE (2006) Perioperative glucose control in the diabetic or nondiabetic patient. South Med J 99:580–589
Hoffman RL, Bartlett EK, Ko C et al (2014) Early discharge and readmission following colorectal resection. J Surg Res 190:579–586
Awad S, Carter S, Purkayastha S et al (2014) Enhanced recovery after bariatric surgery (ERABS): clinical outcomes from a tertiary referral bariatric centre. Obes Surg 24:753–758
Dogan K, Kraaij L, Aarts EO et al (2015) Fast-track bariatric surgery improves perioperative care and logistics compared to conventional care. Obes Surg 25:28–35
Hahl T, Tarkiainen P, Knutar O et al (2016) Outcome of laparoscopic gastric bypass (LRYGB) with a Program for Enhanced Recovery After Surgery (ERAS). Obes Surg 26:505–511
Malone DL, Genuit T, Tracy JK et al (2002) Surgical site infections: reanalysis of risk factors. J Surg Res 103:89–95
Bower WF, Jin L, Underwood MJ et al (2010) Overt diabetes mellitus adversely affects surgical outcomes of noncardiovascular patients. Surgery 147:670–675
Korol E, Johnston K, Waser N et al (2013) A systematic review of risk factors associated with surgical site infections among surgical patients. PLoS ONE 8:e83743
Fearon KC, Ljungqvist O, Von Meyenfeldt M et al (2005) Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 24:466–477
History of the ERAS Society. Retrieved June 24 2016 from http://www.erassociety.org/index.php/about-us/history
Sammour T, Zargar-Shoshtari K, Bhat A et al (2010) A programme of enhanced recovery after surgery is a cost-effective intervention in elective colonic surgery. J N Z Med Assoc 123:61–70
Varadhan KK, Neal KR, Dejong CH et al (2010) The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 29:434–440
Lv L, Shao YF, Zhou YB (2012) The enhanced recovery after surgery (ERAS) pathway for patients undergoing colorectal surgery: an update of meta-analysis of randomized controlled trials. Int J Colorectal Dis 7:1549–1554
Gustafsson UO, Scott MJ, Schwenk W et al (2013) Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg 37:259–284. doi:10.1007/s00268-012-1772-0
Nygren J, Thacker J, Carli F et al (2013) Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg 37:285–305. doi:10.1007/s00268-012-1787-6
Greco M, Capretti G, Beretta L et al (2014) Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 38:1531–1541. doi:10.1007/s00268-013-2416-8
Guyatt GH, Oxman AD, Vist GE et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Gustafsson UO, Scott MJ, Schwenk W et al (2012) Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 31:783–800
Gustafsson U, Hausel J, Thorell A et al (2011) Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 146:571–577
Søreide E, Eriksson LI, Hirlekar G, et al (Task Force on Scandinavian Pre-operative Fasting Guidelines, Clinical Practice Committee Scandinavian Society of Anaesthesiology and Intensive Care Medicine) (2005) Pre-operative fasting guidelines: an update. Acta Anaesthesiol Scand 49:1041–1047
Farrukh A, Higgins A, Singh B et al (2014) Can pre-operative carbohydrate loading be used in diabetic patients undergoing colorectal surgery? Br J Diabetes Vasc Dis 14:102–104
Dhatariya K, Levy N, Kilvert A, Watson B et al (2012) NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med 29:420–433
Laffin MR, Quigley P, Brisebois R et al (2015) Assessment of pre-operative carbohydrate loading and blood glucose concentration in patients with diabetes. Can J Surg 58:S183
Camilleri M (2007) Clinical practice. Diabetic gastroparesis. N Engl J Med 356:820–829
Vittal H, Farrugia G, Gomez G et al (2007) Mechanisms of disease: the pathological basis of gastroparesis—a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol 4:336
Horváth VJ, Izbéki F, Lengyel C et al (2014) Diabetic gastroparesis: functional/morphologic background, diagnosis, and treatment options. Curr Diab Rep 14:527
Fraser R, Horowitz M, Dent J (1991) Hyperglycaemia stimulates pyloric motility in normal subjects. Gut 32:475–478
Kashyap P, Farrugia G (2010) Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years. Gut 59:1716–1726
Frisch A, Chandra P, Smiley D et al (2010) Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 33:1783–1788
King JT Jr, Goulet JL, Perkal MF et al (2011) Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg 253:158–165
Blondet JJ, Beilman GJ (2007) Glycemic control and prevention of perioperative infection. Curr Opin Crit Care 13:421–427
Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non-randomized studies. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.cochrane-handbook.org
Effective Practice and Organisation of Care (EPOC) (2013) How to prepare a risk of bias table for reviews that include more than one study design. EPOC Resources for review authors. Norwegian Knowledge Centre for the Health Services, Oslo. http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed 24 June 2016
Higgins JPT, Altman DG, Sterne JAC (eds). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (eds). Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.cochrane-handbook.org
Effective Practice and Organisation of Care (EPOC) (2015) Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Norwegian Knowledge Centre for the Health Services, Oslo. http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed 24 June 2016
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Haverkamp MP, de Roos MA, Ong KH (2012) The ERAS protocol reduces the length of stay after laparoscopic colectomies. Surg Endosc 26:361–367
Roulin D, Donadini A, Gander S et al (2013) Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg 100:1108–1114
Juul AB, Wetterslev J, Kofoed-Enevoldsen A (2004) Long term postoperative mortality in diabetic patients undergoing major non-cardiac surgery. Eur J Anaesth 21:523–529
Bolognesi MP, Marchant MH Jr, Viens NA et al (2008) The impact of diabetes on perioperative patient outcomes after total hip and total knee arthroplasty in the United States. J Arthroplast 23:92–98
Gil-Bona J, Sabaté A, Pi A et al (2009) Mortality risk factors in surgical patients in a tertiary hospital: a study of patient records in the period 2004–2006. Cir Esp 85:229–237
Jämsen E, Nevalainen P, Eskelinen A et al (2012) Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am 94:e101
Fei Q, Li J, Lin J et al. (2016) Risk factors for surgical site infection following spinal surgery: a meta-analysis. World Neurosurg 95:507–515
Martin ET, Kaye KS, Knott C et al (2016) Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 37:88–99
Luther A, Panteleimonitis S, Kang P, et al (2012) Diabetic patients take longer to recover than non-diabetics within an Enhanced Recovery Programme. In: Poster abstracts. Colorectal Disease 14:12–40
Jorgensen C, Madsbad S, Kehlet H, on behalf of the Lundbeck Foundation Centre for Fast-track Hip and Knee Replacement Collaborative Group (2015) Postoperative morbidity and mortality in type-2 diabetics after fast-track primary total hip and knee arthroplasty. Anesth Analg 120:230–238
Loo FD, Palmer DW, Soergel KH et al (1984) Gastric emptying in patients with diabetes mellitus. Gastroenterology 86:485–494
Wright RA, Clemente R, Wathen R (1985) Diabetic gastroparesis: an abnormality of gastric emptying of solids. Am J Med Sci 289:240–242
Camilleri M, Parkman HP, Shafi MA et al (2013) Clinical guideline: management of gastroparesis. Am J Gastroenterol 108:18–37
Choung RS, Locke GR 3rd, Schleck CD et al (2012) Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol 107:82–88
Kong MF, Horowitz M, Jones KL et al (1999) Natural history of diabetic gastroparesis. Diabetes Care 22:503–507
Horowitz M, Wishart JM, Jones KL et al (1996) Gastric emptying in diabetes: an overview. Diabetes Med 13:S16–S22
Parkman HP, Yates K, Hasler WL et al (2011) Similarities and differences between diabetic and idiopathic gastroparesis. Clin Gastroenterol Hepatol 9:1056–1064
Jones KL, Horowitz M, Wishart MJ et al (1995) Relationships between gastric emptying, intragastric meal distribution and blood glucose concentrations in diabetes mellitus. J Nucl Med 36:2220–2228
Gustafsson UO, Nygren J, Thorell A et al (2008) Pre-operative carbohydrate loading may be used in type 2 diabetes patients. Acta Anaesthesiol Scand 52:946–951
Tryba M, Zenz M, Mlasowsky B et al (1983) Does a stomach tube enhance regurgitation during general anaesthesia? Anaesthesist 32:407–409
Ljungqvist O, Søreide E (2003) Preoperative fasting. Br J Surg 90:400–406
ERAS Compliance Group (2015) The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg 261:1153–1159
Chand M, Tatterton M, Heetun A, et al (2014) Diabetic patients undergoing laparoscopic surgery for colorectal. In: Poster Abstracts. Colorectal Disease 16:37–105
Jia Y, Jin G, Guo S et al (2014) Fast-track surgery decreases the incidence of postoperative delirium and other complications in elderly patients with colorectal carcinoma. Langenbecks Arch Surg 399:77–84
Kobayashi S, Ooshima R, Koizumi S et al (2014) Perioperative care with fast-track management in patients undergoing pancreaticoduodenectomy. World J Surg 38:2430–2437. doi:10.1007/s00268-014-2548-5
Lassen K, Soop M, Nygren J et al (2009) Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 144:961–969
Ahmed J, Khan S, Lim M et al (2012) Enhanced recovery after surgery protocols—compliance and variations in practice during routine colorectal surgery. Colorectal Dis 14:1045–1051
Vlug MS, Bartels SA, Wind J et al (2012) Which fast track elements predict early recovery after colon cancer surgery? Colorectal Dis 14:1001–1008
Nicholson A, Lowe MC, Parker J et al (2014) Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 101:172–188
Oppelstrup H, Ljungqvist O, Thorell A et al (2015) Adherence to the ERAS protocol and 5-year survival after colorectal cancer surgery: a retrospective cohort study [abstract]. J Anesth 62:683–720
American Diabetes Association (2016) 13. Diabetes care in the hospital. Diabetes Care 39(Suppl 1):S99–104
Buchleitner AM, Martínez-Alonso M, Hernández M et al (2012) Perioperative glycaemic control for diabetic patients undergoing surgery. Cochr Database Syst Rev 9: CD007315
Umpierrez GE, Smiley D, Hermayer K et al (2013) Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care 36:2169–2174
Umpierrez GE, Smiley D, Jacobs S et al (2011) Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 34:256–261
Joslin Diabetes Center (2015) Joslin Diabetes Center and Joslin Clinic Guideline for Inpatient Management of Surgical and ICU Patients with Diabetes (Pre-Peri- and Postoperative Care). https://www.joslin.org/docs/Inpatient-management-of-surgical-patients-with-diabetes-_12-30-2015.pdf. Accessed 23 October 2016
Shadish WR, Cook TD, Campbell DT (2001) Experimental and quasi-experimental designs for generalized causal inference. Houghton Mifflin, Boston, xxi: 623p
Kontopantelis E, Doran T, Springate DA (2015) Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ 350:h2750
Goodacre S (2015) Uncontrolled before-after studies: discouraged by Cochrane and EMJ. Emerg Med J 32:507–508
Acknowledgements
We would like to thank Linda Slater, librarian at the University of Alberta, for her assistance in developing the search strategy for this review.
Authors’ contributions
Authors who contributed to the development of this review’s strategy, design, and analysis include ZA, FM, LG, and PS. The two independent reviewers were ZA and ML; FM was the third reviewer who resolved any disagreement. Additional expert opinion on ERAS in reviewing the manuscript was provided by LG. All authors reviewed and agreed on the final manuscript prior to submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Albalawi, Z., Laffin, M., Gramlich, L. et al. Enhanced Recovery After Surgery (ERAS®) in Individuals with Diabetes: A Systematic Review. World J Surg 41, 1927–1934 (2017). https://doi.org/10.1007/s00268-017-3982-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-3982-y